The Ultimate Guide to Resistance Heating Evaporation Coatings
As a classic and widely used thin film deposition technology, resistance heating evaporation coating accurately deposits high-quality thin films on various substrates, continuously driving technological progress and innovation in related fields.
- Low Cost
- Good Film Uniformity
- Higher Film Deposition Rate
- Strong Film-Substrate Bonding
- Compatible With Conductive Materials
Wstitanium Workshop
Our Powerful Facilities

Everything You Should Know About Resistance Heating Evaporation Coating
As one of the most classic and widely used branches of PVD technology, resistance evaporation has penetrated numerous fields, including electronics, optics, mechanics, and medicine. Its advantages, such as simple equipment structure, convenient operation, and controllable costs, have earned it a prominent position in the field of thin film deposition.
What Is Resistance Heating Evaporation?
Resistance heating evaporation involves heating the material to a high temperature in a vacuum using Joule heat generated by an electric current passing through a resistance heating element, transforming it from a solid state into a gas. These gaseous atoms or molecules of the film material freely migrate in the vacuum toward the surface of the substrate (the object being coated). Once they reach the substrate, they gradually deposit and condense, piling up layer by layer to form a uniform and dense film. This is like a precision construction project in the microscopic world. Each atom or molecule is a tiny “brick,” meticulously “assembled” to create a film with specific functions and properties.

Principle of Resistance Heating Evaporation Coating
The core principle of resistance heating evaporation coating is based on the Joule heating effect. According to Joule’s law, when an electric current (I) passes through a resistor (R), heat (Q) is generated, calculated as Q = I²Rt, where t is the time the current flows. In a resistance heating evaporation coating system, current passes through a resistive heating element made of a material with a high melting point, low vapor pressure, and good chemical stability, such as tungsten, molybdenum, tantalum, or non-metallic materials such as high-purity graphite, aluminum oxide ceramic, and boron nitride ceramic. Under the influence of the current, these heating elements generate a large amount of heat due to their inherent resistance, causing their temperature to rise rapidly.
Evaporation and Deposition
As the temperature of the resistance heating element continues to rise, the film material placed on or around it is also gradually heated. When the film material reaches its evaporation temperature, the film material molecules or atoms gain sufficient energy to overcome the intermolecular or atomic forces, transforming directly from solid to gaseous, and evaporation begins. In a vacuum environment, the gaseous film material atoms or molecules diffuse freely in all directions in straight lines. Once gaseous film material atoms or molecules contact the substrate surface, they condense there. They continuously adsorb, migrate, and aggregate on the substrate surface, gradually forming a continuous film.
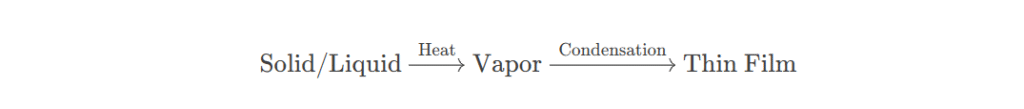
Evaporation Rate
The evaporation rate is affected by factors such as temperature, pressure, and the properties of the material being evaporated. The evaporation rate can be estimated using the Langmuir equation:
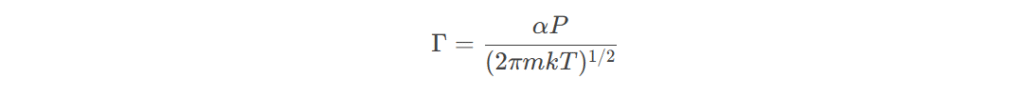
where Γ is the evaporation rate, α is the evaporation coefficient, P is the vapor pressure, m is the molecular mass, k is the Boltzmann constant, and T is the temperature.
Resistor Materials
The Resistor Materials is a key component in resistive heating evaporation coating. Its function is to convert electrical energy into thermal energy through the Joule heating effect, bringing the film material to the evaporation temperature. Therefore, the Resistor Materials must meet core requirements such as a high melting point, low vapor pressure, good electrical conductivity, chemical stability, and compatibility with the film material. Based on the material properties, Resistor Materials can be divided into three categories: metallic, non-metallic, and composite.
Metals

Tungsten (W)
Tungsten has a melting point of 3422°C and can withstand extremely high temperatures without evaporation loss. It is suitable for evaporating low- to medium-melting-point metals. Available materials include tungsten wire (0.5-2mm diameter), tungsten boats (0.1-0.5mm thickness), and tungsten crucibles. However, at high temperatures, it reacts with oxygen and nitrogen to form brittle compounds (such as WO₃ and WN), which can cause the evaporation source to embrittle and fracture. However, it can form alloys with certain metals (such as titanium and zirconium) at high temperatures.

Molybdenum (Mo)
Molybdenum has a melting point of 2617°C and is not prone to chemical reactions with other metals. It is suitable for evaporating copper (Cu), nickel (Ni), and iron (Fe). Available materials include molybdenum boats (5-10mm length, 3-5mm width), and molybdenum wire (0.3-1mm diameter). Molybdenum has greater ductility than tungsten and is compatible with substrates such as glass and ceramics. It is susceptible to oxidation at high temperatures (oxidation begins above 300°C), requiring strict vacuum control (better than 10⁻⁻⁻ Pa); its lifespan is approximately 70% that of tungsten.
Tantalum (Ta)
Tantalum has a melting point of 2996°C. Its combination of high melting point and excellent chemical inertness makes it suitable for evaporating corrosive materials (such as metal halides and oxides). Tantalum boats (commonly used for evaporating fluorides and chlorides) and tantalum foil (0.05-0.2 mm thick) are available. A dense oxide film (Ta₂O₅) easily forms on its surface, enhancing its oxidation resistance. However, its price is relatively high (about 3 times that of tungsten) and it is suitable for high-precision and high-purity coating scenarios.
Non-metallic

Graphite
Graphite has a sublimation temperature of approximately 3650°C, excellent electrical conductivity (resistivity 5-15 μΩ·cm), and is significantly less expensive than metals, making it an ideal choice for evaporating carbon-based materials and metal oxides. Examples include graphite boats and graphite rods. It does not chemically react with most ceramic materials (such as ZrO₂ and SiO₂) and can evaporate high-melting-point oxides. However, it readily reacts with oxygen to form CO₂.

Al₂O₃ & BN & ZrO₂
Aluminum oxide has a melting point of 2072°C, making it suitable for evaporating rare earth oxides. Boron nitride (BN): With a melting point of approximately 3000°C and excellent thermal conductivity (approximately 40 W/(m·K)), it is an ideal crucible material for evaporating metals such as aluminum and copper. Zirconia (ZrO₂): With a melting point of 2715°C and high-temperature shock resistance, it is suitable for evaporating high-melting-point metals (such as molybdenum and niobium).

Composite Materials
Metal-ceramic composite evaporation sources (such as tungsten-alumina and molybdenum-boron nitride) combine the electrical conductivity of metals with the corrosion resistance of ceramics, making them suitable for evaporating complex film materials. For example, an evaporation source with a tungsten core coated with alumina prevents direct reaction between the tungsten and the film while maintaining efficient heating.
Film Materials
Thin film materials are the material foundation for thin films. Their composition, purity, and physical and chemical properties directly determine the film’s functionality (such as conductivity, optics, and wear resistance). Based on their chemical composition, film materials can be categorized into four main categories: metals, alloys, compounds, and organic materials. Let’s now discuss typical thin film materials.

Aluminum (Al)
Aluminum is one of the most commonly used metal film materials. It has a melting point of 660°C, is easily evaporated, and forms uniform thin films. Its thin films offer high reflectivity (approximately 85% in the visible light band), good conductivity (resistivity 2.8 μΩ·cm), and low cost.

Gold (Au)
Gold has a melting point of 1064°C and excellent chemical stability (non-oxidizing and acid and alkali resistant), conductivity (2.4 μΩ·cm), and ductility. Its film reflectivity reaches as high as 98% in the infrared band. However, it is expensive and is often used in combination with nickel (Ni).

Silver (Ag)
Silver has the highest conductivity (1.6 μΩ·cm) and visible light reflectivity (95%) among metals. It has a melting point of 961°C and evaporates easily. It is prone to sulfidation and blackening (forming Ag₂S), requiring a protective layer (such as SiO₂) on the film surface.

Aluminum-Copper (Al-Cu)
Contains 2-5% Cu, which improves the electromigration resistance of aluminum films and is used in integrated circuit interconnects.

Gold-Silver (Au-Ag)
Improves the sulfidation resistance of silver while maintaining high reflectivity, and is used in optical mirrors.

Nickel-Chromium (Ni-Cr)
When Ni accounts for 80% and Cr accounts for 20%, the film has a high resistivity (approximately 100 μΩ·cm) and is used in resistors and strain gauges.
Silicon Dioxide (SiO₂)
Melting point 1713°C, refractive index 1.46 (visible light), and excellent chemical stability make it a common material for optical antireflection coatings and protective films. Graphite or platinum crucibles are required for evaporation.

Zinc Sulfide (ZnS)
Melting point 1830°C, transmittance > 70% in the infrared band (8-12μm), used for infrared window antireflection coatings and laser device coatings. It decomposes easily during evaporation (forming Zn and S), requiring a vacuum of < 10⁻⁴ Pa.

Magnesium Fluoride (MgF₂)
Melting point 1263°C, refractive index 1.38 (visible light), making it an optimal material for antireflection coatings (e.g., eyeglass lenses and camera lenses). It has excellent chemical stability and requires sealed storage.
Advantages of Resistance Heating Evaporation Coating
Low Equipment Cost
Compared to other advanced coating technologies, such as electron beam evaporation and magnetron sputtering, resistance heating evaporation equipment has a relatively simple structure. Therefore, its manufacturing cost is low.
Easy Operation
The operator can precisely adjust the temperature of the resistance heating element by simply controlling the current and voltage of the power supply, thereby controlling the evaporation rate of the film material.
High Film Purity
Because the film material directly transforms from a solid state to a gaseous state during evaporation and then deposits on the substrate surface, chemical reactions and contamination with other substances are avoided. This results in the production of high-quality, high-purity films.
Excellent Film Uniformity
The symmetrical arrangement of multiple evaporation sources ensures that the deposition rate of the gaseous film material atoms or molecules at all locations on the substrate surface is uniform, resulting in a film of uniform thickness.
Strong Film Adhesion
When deposited on the substrate surface, the gaseous film material atoms or molecules interact with the atoms on the substrate surface, forming strong chemical bonds.
Compatibility with Various Materials
Resistance heating evaporation coating is suitable for a wide variety of film materials, including various metals, compounds, and some organic materials.
Conclusion
Resistance heating evaporation, a classic physical vapor deposition technique, plays an irreplaceable role in numerous fields, including electronics, optics, decoration, energy, and biomedicine, thanks to its advantages such as low equipment cost, simple operation, high film purity, and wide material applicability. Its core principle is to utilize the Joule heating effect to evaporate the film material, which is then deposited on the substrate surface under a vacuum to form a functional thin film. The entire process allows precise control of film properties by regulating parameters such as evaporation temperature, vacuum level, and deposition rate.