
Custom Manufacturing Titanium Anode For Chlor-Alkali Industry
Titanium anodes have rapidly become the mainstream anodes in the chlor-alkali industry due to their excellent corrosion resistance, high electrocatalytic activity and long life. Currently, more than 80% of the global chlor-alkali industry uses titanium anode technology.
- Iridium-titanium anode
- Ir - Ta - Ti titanium anode
- Ru - Ir - Ti titanium anode
- Ruthenium-titanium anode (RuO₂-TiO₂)
- Graphite titanium anode
- Customized titanium anode
- Transition metal titanium anode
- Rare earth element titanium anode
Trustworthy Titanium Anode for Chlor-alkali Chemical Industry
As a pillar industry of modern chemistry, the chlor-alkali industry produces chlorine, hydrogen and sodium hydroxide which are widely used in papermaking, textiles, medicine, food, electronics and other fields. Anodes, as the core components of the chlor-alkali electrolysis process, directly determine efficiency, energy consumption and quality. In the early days, the chlor-alkali industry mainly used graphite anodes and lead anodes. Graphite anodes are low-cost, but have poor corrosion resistance and a service life of only 8-12 months. Although lead anodes have good conductivity, they will dissolve during the electrolysis process, pollute the electrolyte and cause the product purity to decrease, and there is also a risk of heavy metal pollution. The excellent corrosion resistance, good conductivity, and efficient electrocatalytic activity of titanium anodes have completely changed the pattern of the chlor-alkali industry and are hailed as a major technological revolution.
Working Principle
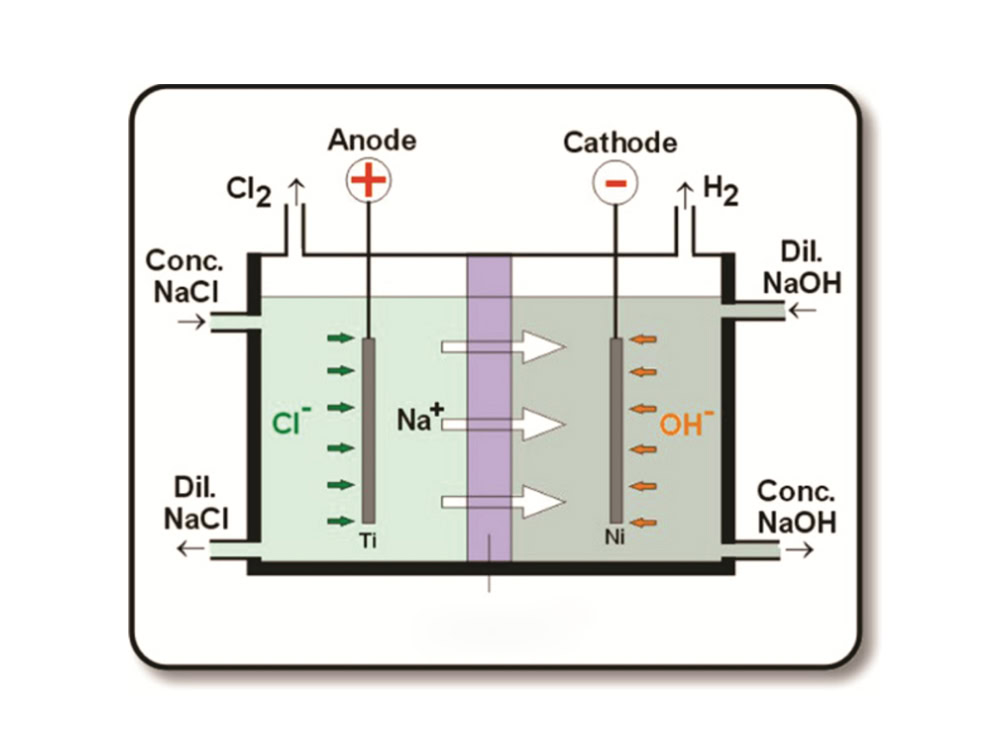
The chlor-alkali industry mainly produces sodium hydroxide (NaOH), chlorine (Cl₂) and hydrogen (H₂) by electrolyzing saturated sodium chloride solution, 2NaCl + 2H₂O =2NaOH + Cl₂↑ + H₂↑. In the chlor-alkali electrolyzer, the titanium anode serves as the electrode for the oxidation reaction. When current passes through the electrolyzer, on the surface of the titanium anode, the chloride ions (Cl⁻) in the solution lose electrons and are oxidized into chlorine (Cl₂), and the electrode reaction formula is: 2Cl⁻ – 2e⁻ = Cl₂↑. The titanium anode itself has good electrical conductivity and can effectively conduct current. At the same time, the metal oxide coating coated on the surface of the titanium anode has excellent electrocatalytic properties, which can reduce the overpotential of the chlorine evolution reaction, thereby reducing the consumption of electrical energy.
Compared with other anodes
The working principle of graphite anode in chlor-alkali electrolysis is also that chloride ions lose electrons on their surface to generate chlorine gas. However, graphite has relatively poor conductivity and is easily corroded and consumed during electrolysis. During electrolysis, the lead anode is converted into lead sulfate and then into lead oxide. Lead oxide is the substance that actually undergoes the oxygen evolution reaction. However, lead sulfate is an insulator, which will hinder electron conduction and increase electrode resistance. Moreover, the electrochemical performance of the lead anode continues to decay, which will not only pollute the electrolyte, but also greatly shorten the life of the electrode.
| Indicator | Titanium Anode | Graphite Anode | Lead Anode |
|---|---|---|---|
| Service Life | 5 – 8 years | 8 – 12 months | 1 – 2 years |
| Current Density | 1.5 – 3 kA/m² | 0.5 – 1 kA/m² | 0.8 – 1.2 kA/m² |
| Chlorine Evolution Overpotential | 800 – 950 mV | 950 – 1100 mV | 900 – 1050 mV |
| Product Purity | > 99.5% | < 98% | Contains heavy metal impurities |
| Energy Consumption | 2200 – 2400 kWh/t Cl₂ | 2800 – 3200 kWh/t Cl₂ | 2500 – 2800 kWh/t Cl₂ |
Titanium Anode Type For Chlor-alkali

Ruthenium titanium anode coating is mainly composed of ruthenium oxide. It has a low chlorine evolution overpotential and can efficiently catalyze the oxidation of chloride ions to generate chlorine gas at a low voltage, thereby reducing the energy consumption of the electrolysis process.

Iridium titanium anode focuses more on working conditions with extremely high requirements for anode stability and corrosion resistance. It still maintains good performance under harsh conditions such as high temperature, high current density and high concentration electrolyte.

Wstitanium has developed a Ru-Ir-Ta-Ti multi-component composite coating. It has achieved the catalytic activity of a variety of metal oxides. It operates stably in a complex impurity environment (such as bromide ions and sulfate ions).
Customized titanium anode manufacturing is the core technology of the chlor-alkali industry. From demand analysis, material selection, manufacturing to quality inspection and after-sales service, each link has an important impact on the performance of the anode and the economic benefits of the enterprise. By deeply understanding the working principle, types and advantages of titanium anodes, practitioners in the chlor-alkali industry can better select and apply titanium anodes.