Customized Titanium Anode for Electrolytic Chlorination
Titanium anode is an insoluble anode made of titanium as a substrate and coated with a specific active coating. Titanium anode has greatly promoted the development of the electrolytic chlorine industry, changed the mode of traditional electrolytic production, improved efficiency and product quality, and reduced costs and environmental pollution.
- Zinc Anode
- Silver Anode
- Nickel Anode
- Copper Anode

Titanium Anode for Chlorine Electrolysis Supplier
In modern industry, chlorine and its related products play a vital role in many fields. From chemical raw material manufacturing, drinking water treatment, papermaking industry to food and sewage treatment, chlorine is used everywhere. Electrolysis is one of the main methods for producing chlorine, and the key lies in the anode. Traditional anode materials have many problems in the electrolysis process, such as short life, high energy consumption, and low efficiency. With the continuous development of materials science, titanium anodes have stood out with their excellent performance and become an ideal choice in the field of electrolytic chlorine.

Ruthenium Titanium Anode
Ruthenium-titanium oxide coating has good electrocatalytic activity, which can reduce the overpotential of chlorine evolution during electrolysis, promote the oxidation reaction of chloride ions, and improve the efficiency of electrolysis. It effectively adsorbs chloride ions and accelerates the reaction rate of their oxidation to chlorine gas.

Iridium Titanium Anode
Iridium titanium anode has received great attention in the field of electrolytic chlorine for its excellent corrosion resistance and stability. The coating is mainly composed of iridium oxide (such as IrO₂). IrO₂ has extremely high chemical stability and good electrocatalytic performance, especially in acidic and strongly oxidizing environments.

Ruthenium Iridium Titanium Anode
The ruthenium-iridium-titanium anode combines the good electrocatalytic activity of the ruthenium-based anode and the excellent corrosion resistance of the iridium-based anode. The ruthenium-iridium-based titanium anode can effectively reduce the overpotential of chlorine evolution and maintain good stability.
Mixed metal oxide titanium anode refers to an anode with a composite coating composed of multiple metal oxides coated on a titanium substrate. In addition to the above-mentioned metal oxides such as ruthenium, iridium, and tantalum, it may also contain precious metal oxides such as platinum, rhodium, and palladium, as well as other transition metal oxides (such as iron, manganese, cobalt, etc.). The combined synergistic effect of these different metal oxides comprehensively improves the electrocatalytic activity, corrosion resistance, conductivity and other properties of the anode. For example, some mixed metal oxide coatings can reduce the overpotential of chlorine evolution while inhibiting the occurrence of side reactions and improving the purity of chlorine. By reasonably adjusting the proportion and structure of each metal oxide in the coating, the adaptability of the anode under different electrolyte compositions and temperature conditions is also optimized.
Working Principle
Chlorine electrolysis is based on the principle of electrolytic cell. In the electrolytic cell, direct current passes through the electrolyte (usually sodium chloride aqueous solution), and oxidation and reduction reactions occur at the anode and cathode respectively. Oxidation reaction occurs at the anode, and the chloride ions (Cl⁻) lose electrons and are oxidized to chlorine gas (Cl₂). Reduction reaction occurs at the cathode, and the hydrogen ions (H⁺) in the aqueous solution gain electrons and are reduced to hydrogen gas (H₂), while producing hydroxide ions (OH⁻), which combine with sodium ions (Na⁺) in the solution to form sodium hydroxide (NaOH). The overall reaction formula is: 2NaCl + 2H₂O → 2NaOH + H₂↑ + Cl₂↑.
Titanium anode plays a key electrocatalytic role in chlorine electrolysis. The active coating on its surface can reduce the overpotential of the chlorine evolution reaction. Overpotential refers to the difference between the potential at which the electrode reaction actually occurs and the potential of the reversible electrode reaction. The presence of overpotential increases the energy consumption of the electrolysis process. The active coating of the titanium anode changes the intermediate steps and activation energy of the reaction, making it easier for chloride ions to lose electrons and be oxidized to chlorine gas on the anode surface. Taking the ruthenium-based titanium anode as an example, during the electrolysis process, chloride ions are first adsorbed on the surface of the RuO₂ coating, and then electron transfer occurs under the action of the electric field to generate adsorbed chlorine atoms (Clads), which further combine to form chlorine gas molecules (Cl₂) and desorb from the anode surface into the solution. This series of reactions can be carried out more efficiently under the catalytic action of the active coating, thereby reducing the energy required for the chlorine evolution reaction.
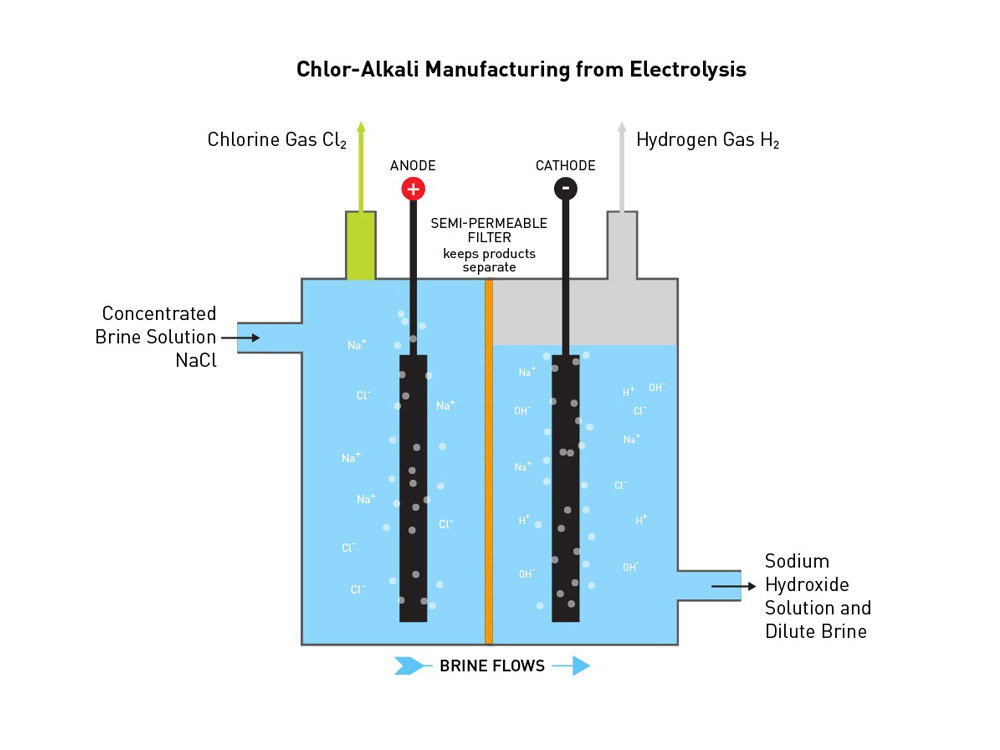
The stability of the titanium anode is due to its unique structure and coating properties. The titanium substrate itself has good mechanical properties and corrosion resistance, and can provide stable support for the active coating. The metal oxide coating on the surface will form a dense passivation film during the electrolysis process. This passivation film can prevent the titanium substrate from directly contacting the electrolyte and prevent the corrosion of titanium. For example, the IrO₂ coating on the surface of the iridium titanium anode will form a stable oxide film on the anode surface during the electrolysis process. The oxide film has good chemical stability and can resist the corrosion of high-concentration chloride ions and strong oxidizing chlorine gas. At the same time, other components in the coating (such as Ta₂O₅, TiO₂, etc.) synergize with IrO₂ to further enhance the stability and protection of the passivation film, so that the iridium titanium anode can maintain stable performance and a long service life during long-term electrolysis.

In the electrolysis of chlorine, the electrode reaction kinetics has an important influence on the electrolysis efficiency and anode performance. The active coating on the surface of the titanium anode can change the kinetic parameters of the electrode reaction, such as the reaction rate constant and the transfer coefficient. By optimizing the composition and structure of the coating, the rate of the electrode reaction can be increased, so that the electrolysis process can reach equilibrium in a shorter time, thereby improving the electrolysis efficiency. In addition, the electrode reaction kinetics is also closely related to factors such as the temperature, concentration, and flow rate of the electrolyte. The titanium anode can adapt to different working conditions to a certain extent. By adjusting its coating performance, it can maintain good electrocatalytic activity and stability in different electrolyte environments, ensuring efficient and stable operation of the electrolysis process.

As the core material in the field of electrolytic chlorine, titanium anode plays an irreplaceable role in the modern chlor-alkali industry and related industries with its unique type and significant advantages. Different types of titanium anodes, such as ruthenium, iridium, ruthenium-iridium and mixed metal oxide titanium anodes, each have different performance characteristics and can meet different working conditions and production needs.