
Custom Manufacturing Titanium Anode for Electrolysis Water
Titanium anodes have shown many advantages in the field of water electrolysis due to their excellent performance, high corrosion resistance, high electrocatalytic activity, long life, and low energy consumption, and have become one of the keys to promoting technological development.
- Iridium-titanium anode
- Ir - Ta - Ti titanium anode
- Ru - Ir - Ti titanium anode
- Ruthenium-titanium anode (RuO₂-TiO₂)
- Graphite titanium anode
- Customized titanium anode
- Transition metal titanium anode
- Rare earth element titanium anode
Titanium Anodes for Electrolysis Water
As a sustainable way to produce hydrogen, water electrolysis has attracted widespread attention. In water electrolysis systems, the performance of electrode materials plays a key role in electrolysis efficiency, energy consumption, stability and life. Titanium anode, as an advanced electrode material, has shown great application potential in the field of water electrolysis due to its unique performance advantages.

Ruthenium oxide coating can effectively reduce the overpotential of chlorine evolution. Although oxygen and hydrogen are mainly evolved in water electrolysis, it has a certain effect on reducing the overpotential of the overall anode reaction, allowing electrolysis to be carried out at a relatively low voltage, thereby saving electricity.
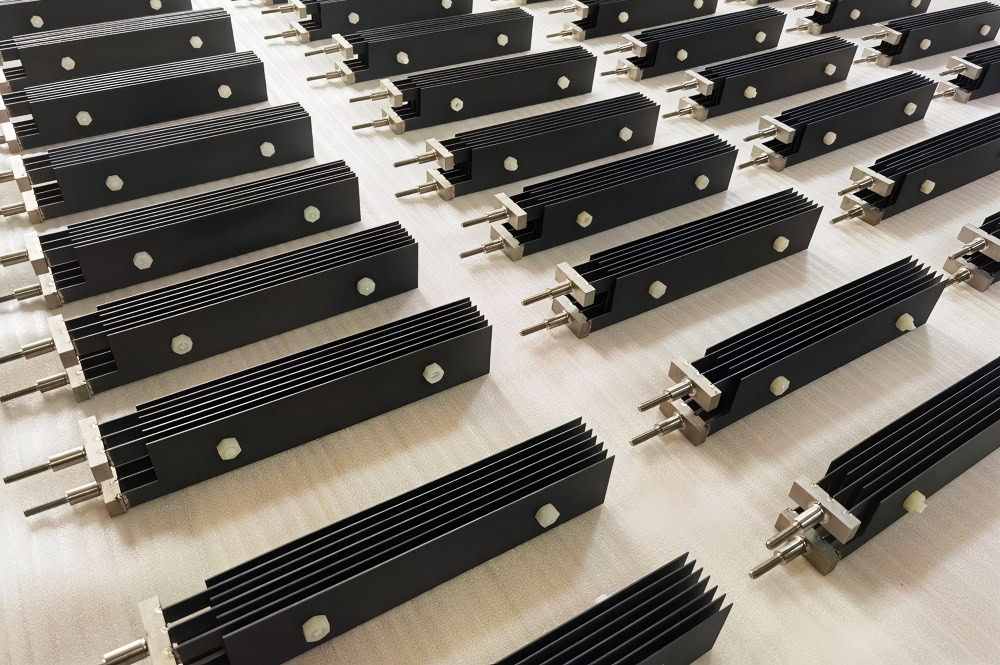
Iridium-coated titanium anode is specially designed for oxygen evolution reaction, significantly reducing the overpotential of oxygen evolution, about 0.2-0.3V. Iridium coating adapts to complex electrochemical environments and is a high-performance anode material commonly used in water electrolysis hydrogen production.
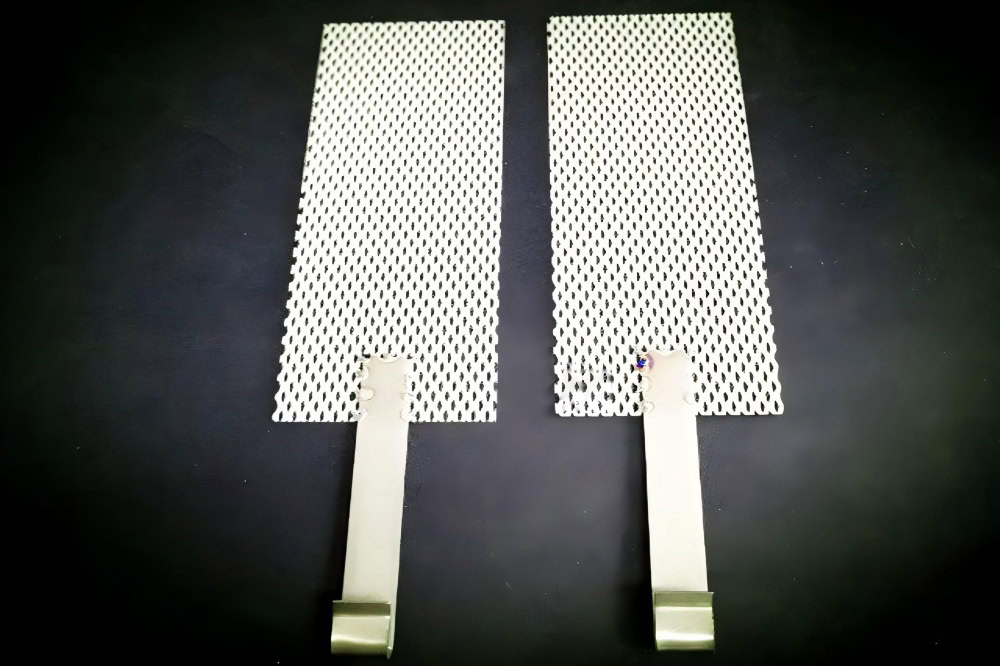
Platinum-coated titanium anode is suitable for precision water electrolysis hydrogen production scenarios with extremely high requirements for electrode performance, such as laboratory research. Accurately control the reaction process to obtain high-quality hydrogen. However, the high cost of platinum limits its large-scale application.
Working Principle
Titanium anode is based on titanium and coated with a layer of electrocatalytically active metal oxide coating (such as platinum, ruthenium, iridium, etc.) on its surface. The titanium substrate has good mechanical strength to ensure the structural stability of the electrode. At the same time, titanium itself is corrosion-resistant and remains relatively stable in a variety of electrolyte environments. The metal oxide coating is the key to the electrocatalytic effect of the titanium anode, giving the titanium anode good conductivity and excellent electrocatalytic performance.
In the electrolysis of water, oxidation reaction occurs at the anode and reduction reaction occurs at the cathode. For the titanium anode, taking the oxygen evolution reaction as an example, when the current passes through the electrolytic cell, the water molecules (H₂O) will undergo an oxidation reaction: 2H₂O – 4e⁻ = O₂↑ + 4H⁺. The active components in the coating can adsorb water molecules, change the electron cloud distribution of water molecules, reduce the activation energy of the reaction, and thus accelerate the reaction rate. At the same time, due to the good conductivity of the coating, the current can be evenly distributed on the anode surface, avoiding local overheating and excessive current density, ensuring that the reaction is carried out under relatively stable conditions, and improving the electrolysis efficiency and the life of the electrode.
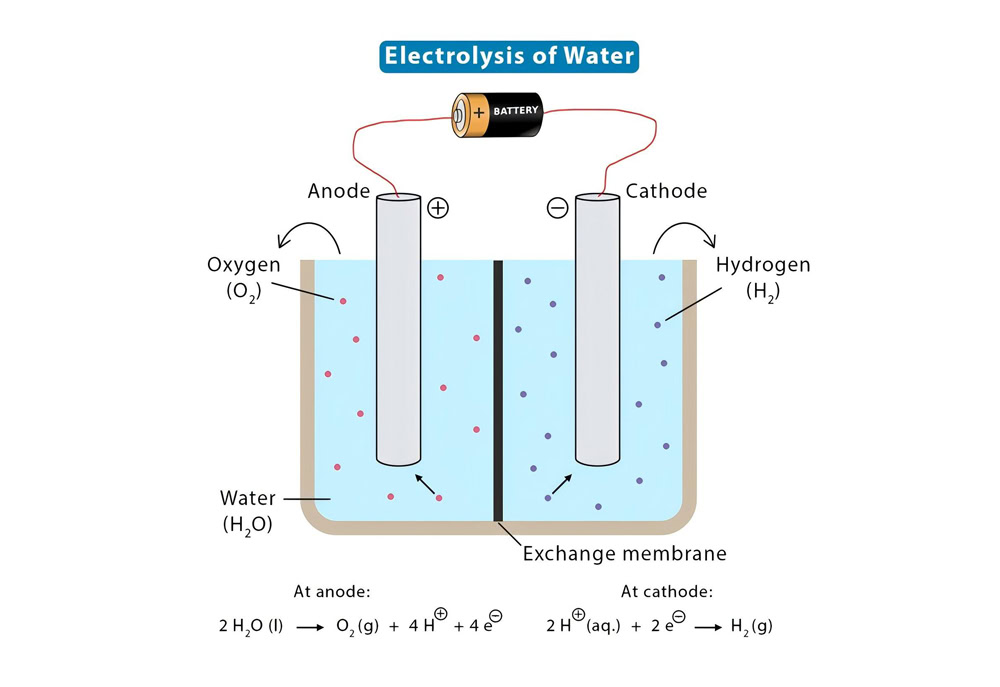
Advantages
- High corrosion resistance
Titanium anodes maintain relatively stable performance in acidic, alkaline, and electrolyte environments containing strong oxidants and strong corrosive ions (such as chloride ions).
- High electrocatalytic activity
Titanium anodes significantly reduce the overpotential of electrode reactions such as oxygen evolution and hydrogen evolution. With the same electrical energy input, more hydrogen and oxygen are produced.
- Reduce energy consumption
Since titanium anodes can reduce the overpotential of electrode reactions. This not only reduces the cost of hydrogen production by electrolysis of water, but also makes it more competitive in the energy market.
- High product purity
Titanium anodes can overcome the problem of dissolution of some traditional anodes (such as graphite anodes and lead anodes), thereby improving the purity of hydrogen and oxygen.
The various types of titanium anodes, such as ruthenium coating, iridium coating and platinum-plated titanium anode, can meet the requirements of electrode performance for different application scenarios and needs. In terms of working principle, the synergistic effect of titanium matrix and metal oxide coating can efficiently catalyze the electrolysis of water. Its advantages such as high corrosion resistance, high electrocatalytic activity, long service life, low energy consumption and ability to improve product purity not only improve the performance and economic benefits of the electrolysis water system, but also provide strong support for the sustainable development of the clean energy industry.