Ultimate Guide to Titanium Anodes For Electrolytic Copper
In the field of electrolytic copper, the application of titanium anode has brought revolutionary changes. It not only solves many problems existing in traditional electrode materials, but also provides strong support for improving the quality and efficiency of electrolytic copper.
- Iridium-coated titanium anode
- Platinum-coated titanium anode
- Ruthenium-coated titanium anode
- Mixed oxide-coated titanium anode
- Titanium electrolytic cell
- Palladium-coated titanium anode
- Carbon-titanium composite anode
- Metal-metal oxide composite anode

Customized Titanium Anodes for Electrolytic Copper Solutions
As a key metal refining technology, electrolytic copper is widely used in many fields such as electronics, electricity, and construction. As one of the core elements of the electrolysis process, the performance of electrode materials directly affects the quality and efficiency of electrolytic copper. Traditional electrode materials, such as graphite anodes and lead anodes, expose many problems in the process of electrolytic copper. The mechanical strength of graphite anodes is low, and they are prone to wear and breakage during the electrolysis process. In addition, the catalytic activity of graphite anodes is low. Lead anodes have dissolution problems, which leads to electrolyte contamination, thereby affecting the purity of cathode copper.

An oxide coating containing iridium (Ir) and tantalum (Ta) is applied to the titanium substrate. Iridium has good chemical stability and high oxygen evolution catalytic activity. Tantalum can enhance the corrosion resistance and mechanical strength of the coating. Iridium Tantalum Titanium anode shows excellent activity in electrolytic copper and significantly reduces the oxygen evolution potential. It has become the preferred electrode material for the production of high-purity electrolytic copper.

A layer of platinum (Pt) is plated on the surface of the titanium substrate. Platinum is a precious metal with extremely high chemical stability and catalytic activity. The platinum-plated titanium anode has an extremely low overpotential and provides efficient and stable electrocatalysis. It is suitable for precision electrolytic copper technology with strict requirements on the quality of copper plating. Due to the high price of platinum, the cost of platinum-plated titanium anode is relatively high.

The lead dioxide titanium anode shows good stability in acidic electrolytes. It can work at higher current densities and has a relatively low cost. The thickness of the lead dioxide coating on one side is generally 0.6mm – 0.8mm, and the size can be customized according to demand, length (100mm – 1.5m) × width (100mm – 1.2m). This anode is suitable for some large-scale electrolytic copper scenarios that are more sensitive to cost and do not have particularly stringent requirements on copper quality.
Titanium Anode for Electrolytic Copper
Titanium anode, full name is titanium-based metal oxide coated electrode (MMO). It consists of two parts, namely titanium substrate and metal oxide coating. A layer of metal oxide coating with electrocatalytic activity is coated on the surface of the titanium substrate.
The titanium substrate usually uses industrial pure titanium Gr1, Gr2, etc. These materials have excellent mechanical strength and corrosion resistance, can maintain stable physical form and mechanical properties in various harsh electrochemical environments, provide solid and reliable support for the surface coating, ensure that the entire electrode will not be deformed or damaged during long-term electrolysis, and ensure the long-term stable operation of the electrode.
The metal oxide coating is the core functional part of the titanium anode. It is coated on the surface of the titanium substrate with precious metal oxides (such as platinum, ruthenium, iridium, etc.) and non-precious metal oxides in a certain proportion. The coating gives the titanium anode good conductivity, high catalytic activity and low oxygen or chlorine evolution overpotential, thereby significantly improving the efficiency of the electrode reaction.

Electrolytic copper working principle
Electrolytic copper is a process that uses electrochemical methods to reduce copper ions from solution to metallic copper and deposit them on the cathode. Copper sulfate solution (CuSO₄) is usually used as the electrolyte. The crude copper to be refined is used as the anode. The pure copper sheet is used as the cathode. When a DC voltage is applied between the two poles, the circuit is closed and current passes through the electrolyte.
At the anode, the copper in the crude copper and other metal impurities (such as iron, zinc, nickel, etc.) will undergo oxidation reactions, lose electrons and enter the solution to become metal ions. Among them, the oxidation reaction of copper is: Cu – 2e⁻ → Cu²⁺. At the cathode, the copper ions (Cu²⁺) in the solution gain electrons and are reduced to metallic copper and deposited on the cathode surface. The reaction formula is: Cu²⁺ + 2e⁻ → Cu. As for other metal ions in the solution, because their standard electrode potential is different from that of copper, under certain electrolytic conditions, their reduction order at the cathode is also different. For example, the standard electrode potential of iron ions (Fe³⁺/Fe²⁺), zinc ions (Zn²⁺), etc. is more negative than that of copper ions. Under normal electrolysis conditions, they are difficult to be reduced at the cathode, and most of them will remain in the solution, thereby achieving the separation of copper from other impurity metals and achieving the purpose of refining copper.
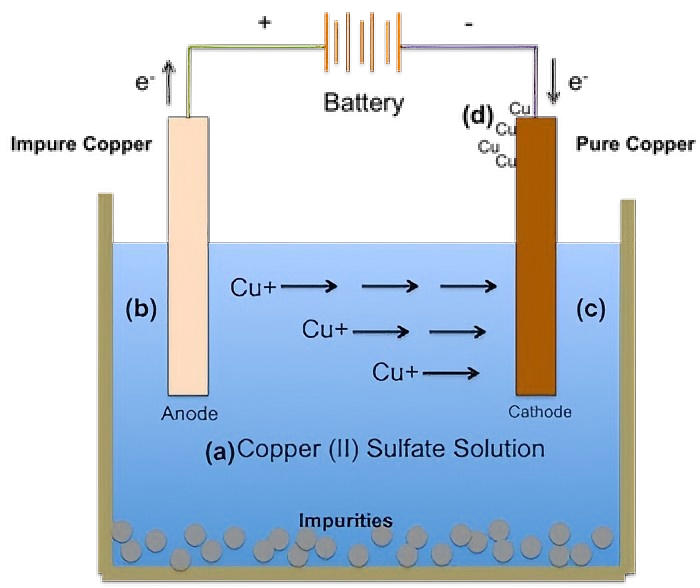
As an insoluble anode, the titanium anode mainly plays the role of conducting electricity and catalyzing the oxygen evolution reaction. The main reaction occurring on the anode surface is the oxidation of water to generate oxygen, and the reaction formula is: 2H₂O – 4e⁻ → O₂↑ + 4H⁺. The metal oxide coating of the titanium anode can provide active sites to accelerate the reaction. Taking the iridium-tantalum titanium anode as an example, the iridium and tantalum oxide coatings on its surface have good catalytic activity for the oxygen evolution reaction, which can reduce the activation energy of the reaction and enable the oxygen evolution reaction to occur smoothly at a lower voltage. The high current efficiency of the titanium anode can enable more electrical energy to be used for the reduction and deposition of copper ions, improve energy utilization efficiency, and reduce production costs.
| Indicator / Anode Type | Traditional Lead Anode | Ruthenium – Titanium Anode | Plated Platinum – Titanium Anode |
| Cathode Copper Purity | 99.90% | Above 99.99% | Above 99.999% |
| Electrolysis Efficiency Improvement Percentage | – | 20% | 18% |
| Electrode Service Life (months) | 3 | 24 | 18 |
| Unit Output Energy Consumption Reduction Percentage | – | 15% | 13% |
| Product Yield Rate | 80% | 92% | 95% |
It can be clearly seen from the above data that titanium anodes have obvious advantages over traditional lead anodes in electrolytic copper applications. In terms of purity, iridium-tantalum-titanium anodes and platinum-plated titanium anodes can greatly improve the purity of cathode copper to meet the needs of different high-end fields. In terms of electrolysis efficiency, both titanium anodes significantly increase output. The extension of electrode life reduces production interruption time; the reduction in energy consumption saves companies a lot of costs. The improvement of product yield directly increases the economic benefits of the company. These data strongly prove the application value and broad prospects of titanium anodes in the electrolytic copper industry.
Conclusion
Titanium anodes have shown great advantages and application potential in the field of electrolytic copper. Iridium-tantalum-titanium anodes, platinum-plated titanium anodes, lead dioxide-titanium anodes, etc., meet diverse production needs with their own characteristics. However, titanium anodes also face challenges such as high costs and high technical requirements in the process of promotion and application. Looking to the future, titanium anodes will continue to develop in the direction of coating material innovation, intelligence and automation, green and sustainable development, and multifunctionality, providing strong support for the technological progress and sustainable development of the electrolytic copper industry.