Titanium Anodes for Wastewater Treatment
Titanium anode has excellent corrosion resistance, high conductivity, good catalytic activity and long service life. It can operate stably in harsh sewage environment, providing a solid foundation for the application of electrochemical sewage treatment technology.
- Iridium-coated titanium anode
- Platinum-coated titanium anode
- Ruthenium-coated titanium anode
- Mixed oxide-coated titanium anode
- Titanium electrolytic cell
- Palladium-coated titanium anode
- Carbon-titanium composite anode
- Metal-metal oxide composite anode

Custom Manufacturing of Titanium Anodes For Wastewater Treatment
With the acceleration of global industrialization and urbanization, water pollution is becoming increasingly serious. Sewage treatment, as a key link to ensure the sustainable use of water resources and maintain the balance of the ecological environment, has received unprecedented attention. Titanium anodes, as the core components of electrochemical sewage treatment systems, play a decisive role in the efficiency and effectiveness of sewage treatment. Titanium-based coated anode is a titanium substrate with one or more layers of catalytically active metal oxide coatings coated on the surface through a specific process. This type of anode is most widely used in sewage treatment. According to the different coating compositions, it can be further divided into the following types.
The surface is coated with an oxide coating of ruthenium (Ru) and iridium (Ir). The ruthenium-iridium coating has excellent chlorine evolution catalytic performance. In sewage containing chloride ions, it can efficiently promote the oxidation of chloride ions to generate chlorine gas, and then generate hypochlorous acid and hypochlorite ions with strong oxidizing properties, which can oxidize and degrade organic matter, bacteria, viruses and other pollutants in sewage.

The surface coating is lead dioxide (PbO₂), which is divided into two crystal forms: α-PbO₂ and β-PbO₂. β-PbO₂ has high electrocatalytic activity and stability, and has strong oxidation ability for organic pollutants. When treating industrial wastewater containing a large amount of difficult-to-degrade organic pollutants such as printing and dyeing wastewater and pharmaceutical wastewater, the titanium-based lead dioxide coated anode has shown good treatment effects.

Titanium-based multi-component composite coating anode. Such as titanium-based ruthenium-iridium-tin (Ru-Ir-Sn), titanium-based iridium-tantalum (Ir-Ta) and other composite coatings. It combines the advantages of multiple single coating anodes, such as excellent oxygen and chlorine evolution catalytic performance, adapting to the treatment needs of different types of sewage, and showing unique advantages in the treatment of sewage with complex water quality.
Working Principle
In electrochemical sewage treatment, the titanium anode acts as an anode and undergoes an oxidation reaction. Taking the treatment of organic pollutants as an example, when the organic molecules in the sewage approach the surface of the titanium anode, the electrons in the molecules are acted upon by the anode electric field, and an oxidation reaction occurs.
These organic molecules are first oxidized into free radical intermediates, which have high reactivity and can further react with water or other substances, and are gradually oxidized and decomposed into carbon dioxide, water and other small inorganic molecules. For example, when treating methanol (CH₃OH), methanol loses electrons on the surface of the titanium anode and undergoes an oxidation reaction: CH₃OH + H₂O – 6e⁻ = CO₂ + 6H⁺. For sewage containing chloride ions, titanium anodes (such as titanium-based ruthenium-iridium coated anodes) promote the oxidation reaction of chloride ions (Cl⁻). Chloride ions lose electrons on the anode surface to generate chlorine gas (Cl₂): 2Cl⁻ – 2e⁻ = Cl₂↑. The generated chlorine will react with water to generate hypochlorous acid (HClO) and hypochlorite ions (ClO⁻): Cl₂ + H₂O ⇌ HClO + H⁺ + Cl⁻. HClO and ClO⁻ are highly oxidizing and can oxidize and degrade pollutants such as organic matter, bacteria, and viruses in sewage.
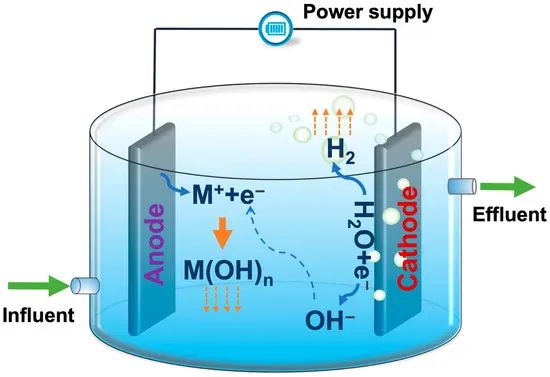
The titanium anode and cathode together form a complete electrolysis circuit. Taking the removal of heavy metal ions in sewage as an example, while the organic matter at the anode is oxidized, the heavy metal ions obtain electrons at the cathode and are reduced to metal elements and deposited on the cathode surface. For example, when treating sewage containing copper ions (Cu²⁺), the cathode reaction is: Cu²⁺ + 2e⁻ = Cu. The cathode and cathode reactions work together to remove a variety of pollutants in sewage and achieve the purpose of purifying water quality.
Conclusion
As the core component of electrochemical sewage treatment technology, titanium anode has shown broad application prospects in the field of sewage treatment due to its unique performance and advantages. Although titanium anode has many advantages in sewage treatment, it still faces some challenges. For example, some high-performance titanium anodes (such as titanium-based precious metal anodes) are expensive, which limits their large-scale application. For some special types of sewage, such as sewage containing a large amount of suspended matter and high concentration of salt, it is necessary to further optimize the performance and technical parameters of titanium anodes to improve the treatment effect.