Zinc Sacrificial Anode Manufacturer and Supplier In China
Wstitanium makes greater contributions to the global metal corrosion protection industry with its advanced technology, strict quality control and continuous innovation in the field of zinc sacrificial anode manufacturing.
- Pure Zinc Anode
- Customized Zinc Anode
- Marine Facility Zinc Anode
- Zinc-Aluminum-Cadmium Anode
- Strip Zinc Anode
- Rod Zinc Anode
- For Chemical
- For Mhttps://wstitanium.com/marine-growth-prevention-systems-anodes-manufacturer-and-supplier/arine

Reputable Zinc Sacrificial Anode Factory - Wstitanium
In the field of metal corrosion resistance, sacrificial anode cathodic protection is an extremely important and widely used technology, and zinc sacrificial anodes occupy a prominent position among many sacrificial anode materials due to their good electrochemical properties, moderate prices and stable chemical properties. As a backbone enterprise involved in the manufacture of zinc sacrificial anodes, Wstitanium has mastered advanced manufacturing technology and is able to produce high-quality, high-performance zinc sacrificial anodes to meet the needs of different industries and application scenarios.

Zinc-Aluminum-Cadmium Anode
Suitable for seawater and chloride-containing environments, common models are ZE series, etc. The ambient temperature of use should not exceed 50℃.

Pure Zinc Anode
Does not contain other alloy elements, has lower electrode potential and higher current efficiency, and is suitable for various seawater temperature and resistivity conditions.

Marine Facility Zinc Anode
Used for anti-corrosion protection of marine engineering facilities such as ports, docks, and offshore platforms, common models include ZI series.

Tank Zinc Anode
Used for corrosion protection of large storage tanks, common models are ZC series.

Hull Zinc Anode
Specially used for corrosion protection of ships, common models are ZH series.

Underground Pipeline Zinc Anode
Used for corrosion protection of buried metal pipelines, common models are ZP series.

Cooling Water System Zinc Anode
Used for corrosion protection of related metal parts in seawater cooling water system, common models are ZE series.

Ship Tanks Zinc Anode
Used for corrosion protection of ship tanks and other parts, common models are ZT series.

Welded Zinc Anode
Connected to the protected metal structure by welding, with stronger connection strength and stability.

Bolted Zinc Anode
Connected to the protected metal structure by bolts and nuts, easy to install and disassemble.
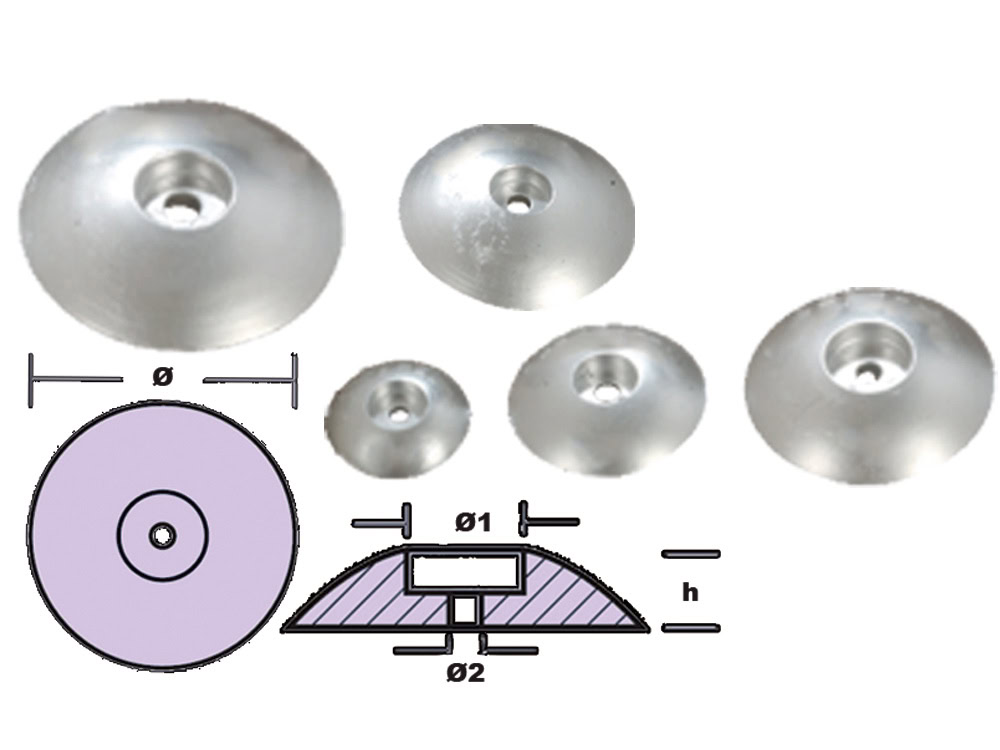
Disc Zinc Anode
In the shape of a disc, suitable for large protection areas.

Strip Zinc Anode
Has a large surface area and flexible installation method, models such as ZR-1 and ZR-2.
Working Principle of Zinc Sacrificial Anode
Metal corrosion is essentially an electrochemical process. When metal comes into contact with an electrolyte solution, due to the microscopic heterogeneity of the metal surface, a potential difference will be formed at different locations, thus forming countless tiny primary cells. In these primary cells, the location with a lower potential becomes the anode, where an oxidation reaction occurs, and metal atoms lose electrons and become metal ions that enter the solution, causing metal corrosion; the location with a higher potential acts as the cathode, where a reduction reaction occurs, and the oxidizing substances in the solution gain electrons. For example, when steel is in humid air, iron is oxidized as the anode, and the reaction formula is: Fe−2e−⟶Fe2+. The reduction reaction of oxygen occurs at the cathode: O2+2H2O+4e−⟶4OH−
Protection Principle of Zinc Sacrificial Anode
Zinc sacrificial anode protection is based on the above electrochemical corrosion principle. By connecting the zinc sacrificial anode to the protected metal structure, a new galvanic cell is formed between the two in the electrolyte solution. Since the electrode potential of zinc is more negative than that of most protected metals (such as steel), in this new galvanic cell, zinc becomes the anode, undergoes oxidation reaction first, and is continuously consumed by corrosion; while the protected metal becomes the cathode, obtains electrons provided by the zinc anode, and the corrosion process on its surface is suppressed, thereby achieving the purpose of corrosion protection.The anode reaction formula is:Zn−2e−⟶Zn2+. The electrons flow to the protected metal, making it difficult for the protected metal to undergo oxidation reaction, thus achieving protection.
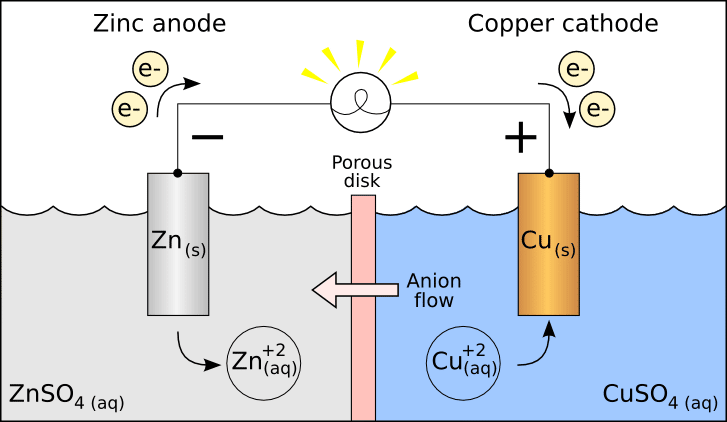
Electrode Potential and Potential Difference
Electrode potential is a physical quantity that measures the tendency of a metal to lose or gain electrons in an electrolyte solution. Different metals have different standard electrode potentials. The more negative the standard electrode potential, the easier it is for the metal to lose electrons and the higher the chemical activity. The standard electrode potential of zinc is -0.76V (relative to the standard hydrogen electrode), which has a significant potential difference from steel (the electrode potential is about -0.44V). In the sacrificial anode protection system, this potential difference is crucial, and it is the driving force for the generation of protection current. The larger the potential difference, the larger the protection current generated, and the better the protection effect, but it may also cause the anode to consume too quickly. Therefore, in practical applications, it is necessary to seek a balance between the protection effect and the service life of the anode to ensure the economy and effectiveness of the system.
Performance And Characteristics of Zinc Sacrificial Anode
The potential of the zinc sacrificial anode is stable and moderate, and the driving voltage is low, about 0.25V, to avoid over-protection. It has a high current efficiency of over 65%, which can efficiently convert chemical energy into electrical energy and extend its service life. At the same time, it has good corrosion resistance and can adapt to various environments such as soil and seawater. It has a low melting point and can be easily made into different shapes to meet engineering needs. In addition, the zinc sacrificial anode is more environmentally friendly, pollution-free during use, and can be recycled and reused after being discarded.
- Stable Potential Output
The zinc sacrificial anode can provide relatively stable potential output in common electrolyte environments, such as seawater and soil. Its working potential is generally between -1.05V and -1.10V (relative to the saturated copper sulfate reference electrode). This stable potential ensures a continuous and stable supply of protection current, thereby providing reliable protection for the protected metal.
- High Current Efficiency
In electrolytes such as seawater, the current efficiency of zinc sacrificial anodes can usually reach more than 85%. This means that in practical applications, most of the current passing through the anode can be effectively used to protect the protected metal, reducing the ineffective consumption of the anode and improving the utilization efficiency of the anode.
- Good Casting Performance
Zinc has good casting performance and is easy to process into anodes of various shapes and sizes. This makes it possible to flexibly design and manufacture zinc sacrificial anodes of different specifications according to the characteristics of the protected metal structure and the protection requirements in practical applications, meeting a variety of engineering application scenarios.
- Moderate Density
The density of zinc is 7.14g/cm³, which is relatively moderate. Compared with some metals with higher density, zinc sacrificial anode is lighter and easier to transport and install under the same protection effect; compared with metals with lower density, it provides more protection per unit volume, and can provide more lasting protection in a limited space.
- Good Corrosion Resistance
A relatively dense corrosion product film will form on the surface of the zinc sacrificial anode. This film can slow down the further corrosion of the anode to a certain extent, improve the chemical stability of the anode, and extend the service life of the anode. At the same time, this corrosion product film also has a certain conductivity and will not significantly hinder the transmission of the protective current.
- Environmentally Friendly
Zinc is a relatively environmentally friendly metal. In the natural environment, its corrosion products have less pollution to the soil, water and other environments. Compared with some sacrificial anode materials containing heavy metals, zinc sacrificial anodes will not cause serious harm to the environment during use, which meets modern environmental protection requirements.
Custom Manufacturing Zinc Sacrificial Anode Solutions
Wstitanium’s professional team will communicate with you in depth to learn more about the specific application scenarios of zinc sacrificial anodes. Different application scenarios have different corrosion environment characteristics. For example, the seawater environment has high salinity and rich microorganisms. The underground soil environment has large differences in pH and resistivity. These factors directly affect the design and manufacturing requirements of zinc sacrificial anodes.
According to the application scenario, we will assist you in determining key technical indicators, including the required anode potential range, current output size, service life expectation, size specification restrictions, etc. For example, for long-distance oil pipelines, larger-sized, long-life zinc sacrificial anodes that can adapt to different soil environments may be required. For local protection of small ships, more attention may be paid to the compact size and ease of installation of the anode.

Design Scheme
Based on the needs and application environment, Wstitanium’s R&D team will accurately design the alloy composition of the zinc sacrificial anode. Adding an appropriate amount of aluminum (Al) to zinc can refine the grains and improve the strength and corrosion resistance of the anode. The aluminum content is generally controlled at 0.1-0.5%. Adding cadmium (Cd) can enhance potential stability, and the content is usually 0.05-0.15%. At the same time, according to special environmental requirements, such as high temperature and high acid and alkali environment, other trace elements will be considered to optimize the anode performance.
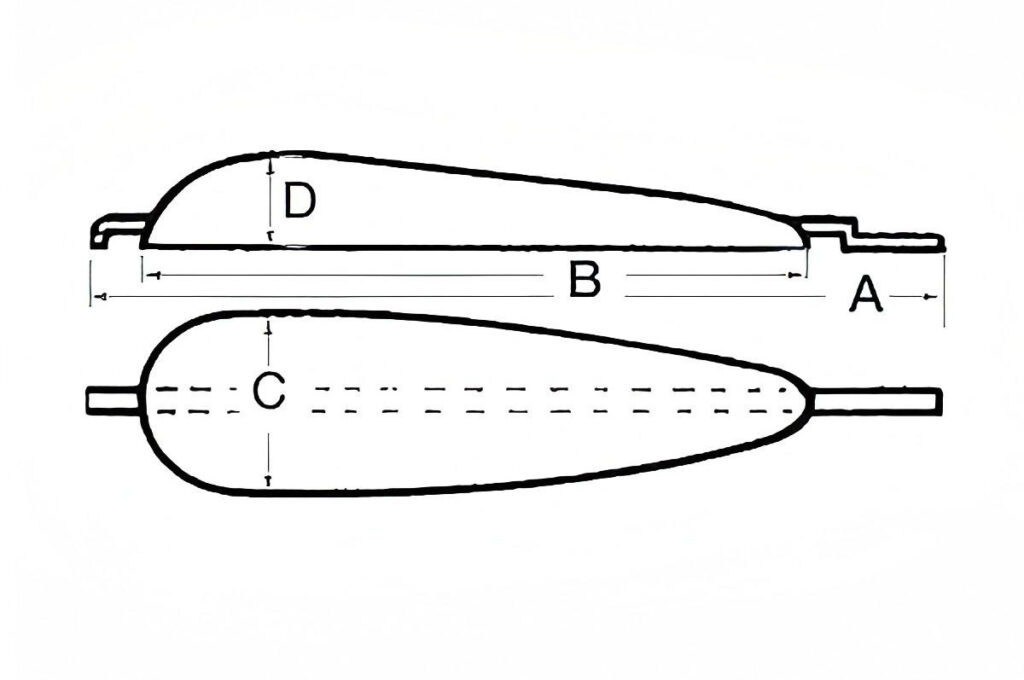
Size and Shape
Design the size and shape of the zinc sacrificial anode according to the structural characteristics of the protected metal and the installation space. Common shapes include cylindrical, block, strip, etc. For pipelines, strip anodes can be tightly wound to achieve uniform protection; for large steel structures, block anodes can be flexibly arranged according to the corrosion risk area. In terms of size, factors such as protection current demand and anode consumption rate will be comprehensively considered to ensure that the anode provides sufficient protection current during its service life.

Raw Materials
The purity of the zinc raw materials used by Wstitanium is crucial, and the purity is usually required to reach more than 99.9%. High-purity zinc can ensure that the anode has stable and good electrochemical properties. Fillers are used to improve the physical properties and processing properties of the anode. Wstitanium usually uses graphite powder, titanium dioxide, etc. as fillers. Graphite powder has good conductivity, which can enhance the electron conduction inside the anode and improve the working efficiency of the anode. Titanium dioxide can improve the forming performance of the anode, making it easier to obtain precise shapes and sizes during the casting process, and at the same time it can also improve the corrosion resistance of the anode to a certain extent.
Smelting
Wstitanium uses an advanced medium frequency induction furnace to melt zinc raw materials. During the smelting process, the temperature and time are strictly controlled. Generally, the temperature is controlled between 450-500°C. The smelting time is determined according to the amount of raw materials and the power of the equipment, usually 1-2 hours. Accurate temperature control is essential to ensure the uniform distribution of alloying elements and the quality of the anode. Too high a temperature may cause the alloying elements to burn out and affect the anode performance; too low a temperature may prevent the alloying elements from being fully dissolved and evenly dispersed.

After the zinc raw material is smelted to the predetermined temperature, alloying elements such as aluminum, cadmium, magnesium, additives and fillers are added in turn according to precise proportions. Through strong stirring, these elements and materials are fully dissolved and evenly dispersed in the zinc liquid. The stirring time is generally 20-30 minutes to ensure the alloying effect. During the stirring process, the composition and temperature changes of the zinc liquid must be closely monitored, and the process parameters must be adjusted in time to ensure that the alloy composition meets the design requirements.
Mold Design and Manufacturing
Wstitanium carefully designs and manufactures high-precision molds according to different anode specifications and shape requirements. The mold material is usually heat-resistant and wear-resistant alloy steel to ensure the dimensional accuracy and stability of the mold during high-temperature casting. The design of the mold fully considers factors such as heat dissipation and demoulding of the anode, and adopts reasonable cooling channels and demoulding structures to ensure that the surface quality of the cast anode is good, without defects such as pores and sand holes.

The molten zinc alloy liquid is cast into the mold by gravity casting or low-pressure casting. Gravity casting is suitable for the manufacture of anodes with simple shapes and large sizes, and the cost is low. Low-pressure casting is more suitable for the manufacture of anodes with complex shapes and high dimensional accuracy requirements, which can effectively reduce defects such as pores and shrinkage holes in castings. During the casting process, parameters such as casting temperature, casting speed and cooling speed are strictly controlled. The casting temperature is generally controlled between 430-470°C, the casting speed is determined according to the mold structure and the anode size, and the cooling speed is adjusted by the cooling system of the mold to ensure that the crystal structure of the anode is uniform and dense.

The anode blank after casting needs to be machined to achieve precise size and surface quality requirements. Wstitanium uses advanced CNC machining equipment to perform cutting, drilling, grinding and other processing operations on the anode. For example, the anode is cut into a predetermined length and width by a CNC cutting machine, and the mounting hole is drilled on the anode by a CNC drilling machine to ensure that the position accuracy and size accuracy of the hole meet the design requirements. Grinding is used to remove burrs and oxide scales on the surface of the anode, so that the surface of the anode is smooth and flat, and the appearance quality and corrosion resistance of the anode are improved.

Zinc sacrificial anode quality inspection
Wstitanium’s series of quality inspections for zinc sacrificial anodes include:
Chemical Composition
Wstitanium invests in advanced spectrometers to accurately test the chemical composition of zinc sacrificial anodes. This equipment can quickly and accurately analyze the content of major elements such as zinc, aluminum, cadmium, magnesium, and other trace elements. During the manufacturing process, each batch of raw materials and finished anodes is strictly tested for chemical composition to ensure that they meet design requirements and relevant standards. For example, for zinc content, the test results are required to be between 99.9% – 99.95%; aluminum content is controlled between 0.1% – 0.3%; cadmium content is controlled between 0.05% – 0.15%; magnesium content is controlled between 0.01% – 0.05%.

Hardness
The hardness of the anode is tested using a hardness tester to evaluate the mechanical properties and processing performance of the anode. The hardness test adopts the Rockwell hardness or Brinell hardness test method, and the corresponding hardness range is specified according to different anode specifications and application requirements. Generally speaking, the hardness of zinc sacrificial anodes should be between 50 – 80HRB (Rockwell hardness) or 40 – 60HBW (Brinell hardness). Failure to meet the hardness requirements may affect the installation and use of the anode, such as deformation and damage during installation.

Open circuit potential
Use a reference electrode and a potential meter to measure the open circuit potential of the anode to evaluate the activity and initial potential state of the anode. The open circuit potential is the potential of the anode when it is not connected to the protected metal. For zinc sacrificial anodes, its open circuit potential is generally between -1.05V – -1.15V (relative to a saturated copper sulfate reference electrode).
Working potential
Simulate the actual working state of the anode, measure its working potential after being connected to the protected metal, and judge the potential stability of the anode when providing a protective current. The working potential should be stable within the design requirements, generally -0.85V – -1.20V (relative to a saturated copper sulfate reference electrode). Unstable working potential may lead to poor protection effect and even corrosion of the protected metal.
Current Efficiency
The current efficiency of the anode is calculated by measuring the amount of electricity consumed by the anode in a certain period of time and the actual protection current provided by the anode through a special current test device. Current efficiency is an important indicator to measure the performance of the anode. The current efficiency of the zinc sacrificial anode manufactured by Wstitanium is usually required to reach more than 85%. Too low current efficiency will cause the anode to be consumed too quickly, shorten its service life, and increase maintenance costs.
Zinc sacrificial anode application
Zinc sacrificial anode plays an indispensable role in metal corrosion protection in many fields due to its simplicity, effectiveness, economy and practicality. With the development of science and technology and the improvement of corrosion protection requirements, its application prospects will be broader.
Offshore Drilling Platform
A large offshore drilling platform uses zinc sacrificial anodes made by Wstitanium for cathodic protection. A large number of zinc sacrificial anodes are distributed in the main structure, pile legs, and jackets of the platform. During use, regular monitoring found that the protection effect of the anode is good, and the potential of the protected metal is always kept within a reasonable protection range. After years of operation, the corrosion of the metal structure of the platform has been significantly reduced, and there have been no structural safety problems caused by corrosion, which effectively guarantees the normal operation and service life of the drilling platform, and greatly reduces the maintenance cost and the risk of shutdown.

Submarine Pipeline
A long-distance submarine oil pipeline uses Wstitanium’s zinc sacrificial anodes. Due to the complex submarine environment and the strong corrosiveness of seawater, the performance requirements for the anodes are extremely high. Before the pipeline is laid, the specifications and distribution plan of the anodes are precisely designed according to factors such as the length, diameter, and seawater characteristics of the sea area where the pipeline is located. During operation, the working status of the anodes and the protection of the pipeline are monitored in real time through an intelligent monitoring system. Over the years, the corrosion of the pipeline has been effectively controlled, and no accidents such as leakage have occurred, ensuring the safe transportation of oil.

Large Commercial Ship
A large commercial ship with a load of hundreds of thousands of tons selected zinc sacrificial anodes manufactured by Wstitanium during its construction. Anodes were installed on the ship’s hull, rudder blades, propellers and other parts to effectively prevent seawater from corroding the ship’s metal structure. During the long-term voyage of the ship, the anodes were regularly inspected and maintained, and replaced in time according to the consumption of the anodes. After many ocean voyages, the ship’s metal structure remained in good condition without serious corrosion problems, ensuring the ship’s navigation safety and service life.

Marine Research Vessel
A marine research vessel has extremely high requirements for the reliability and stability of its equipment, because it is often in a harsh marine environment when performing scientific research missions. The ship uses Wstitanium’s high-performance zinc sacrificial anodes, combined with advanced coating protection technology, to provide all-round protection for the hull. In many years of scientific research missions, the anode and coating protection system work together to effectively resist the corrosion of seawater and the attachment of marine organisms, ensuring the normal operation of the research vessel and providing a reliable platform for scientific research.

Bridge
In a cross-sea bridge project, Wstitanium provided customized zinc sacrificial anodes for the underwater foundation of the bridge. In view of the high corrosiveness and tidal effects of the seawater environment, a special anode shape and fixing method were designed to ensure that the anode can work stably in harsh environments. After long-term monitoring, the corrosion of the steel structure of the bridge foundation has been effectively suppressed, extending the service life of the bridge and ensuring safe and smooth traffic.

Wstitanium’s technical strength, service capabilities and innovative ideas in the customized manufacturing of zinc sacrificial anodes work together to create high-quality solutions for metal corrosion protection, meet the diverse needs of different industries, and promote the sustainable development of the industry.