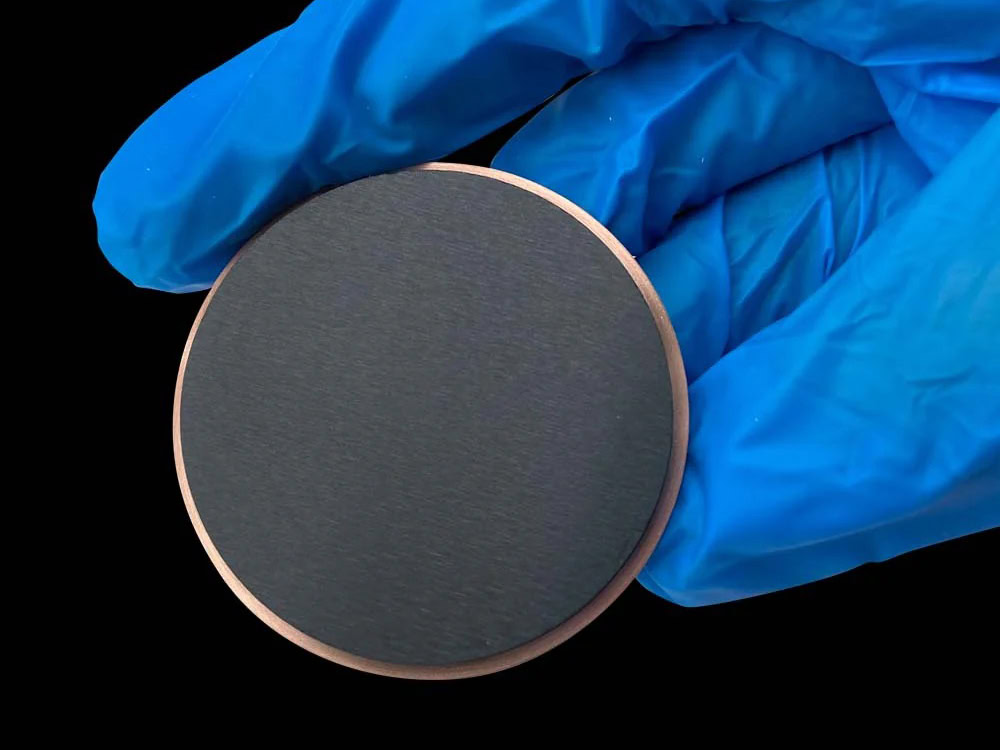
MMO Titanium Anode For Metallurgy
Certified: CE & SGS & ROHS
Shape: Requested
Diameter: Customized
Drawings: STEP, IGS , X_T, PDF
Shipping: DHL, Fedex, or UPS & Ocean Freight

20+ YEARS EXPERIENCE SENIOR BUSINESS MANAGER
Ask Michin For What You Want?
Hydrometallurgy, a core technology for extracting metals such as copper, nickel, cobalt, and zinc, has become a mainstream trend in the modern metallurgical industry due to its low energy consumption, high selectivity, and environmental friendliness. The MMO titanium anode substrate can withstand extremely corrosive environments such as 98% concentrated sulfuric acid and 50% hydrochloric acid.
The MMO coating precisely regulates the electrochemical reaction, maintaining low overpotential and low losses even at high current densities, making it perfectly suited to the demanding hydrometallurgical operating conditions of strong acid, high salt, and high current. Since its initial application in copper electrorefining in the 1980s, MMO titanium anodes have gradually penetrated into leachate purification, metal electrowinning, electrorefining, and precious metal recovery.
| Technical Measurement | Performance |
| Coating Element | Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂),Platinum |
| Substrate Material | Titanium Gr1 or Gr2 |
| Titanium Anode Shape | Customized Plate/Mesh/Tube/Rod/Wire/Disc |
| Coating Thickness | 8~20 μm |
| Coating Uniformity | 90% min. |
| Current Density | ≤ 20000 A/m² |
| Operating Voltage | ≤ 24V |
| PH Range | 1~14 |
| Temperature | < 80 °C |
| Fluoride Ion Content | < 50 mg/L |
| Warranty | More than 5 years |
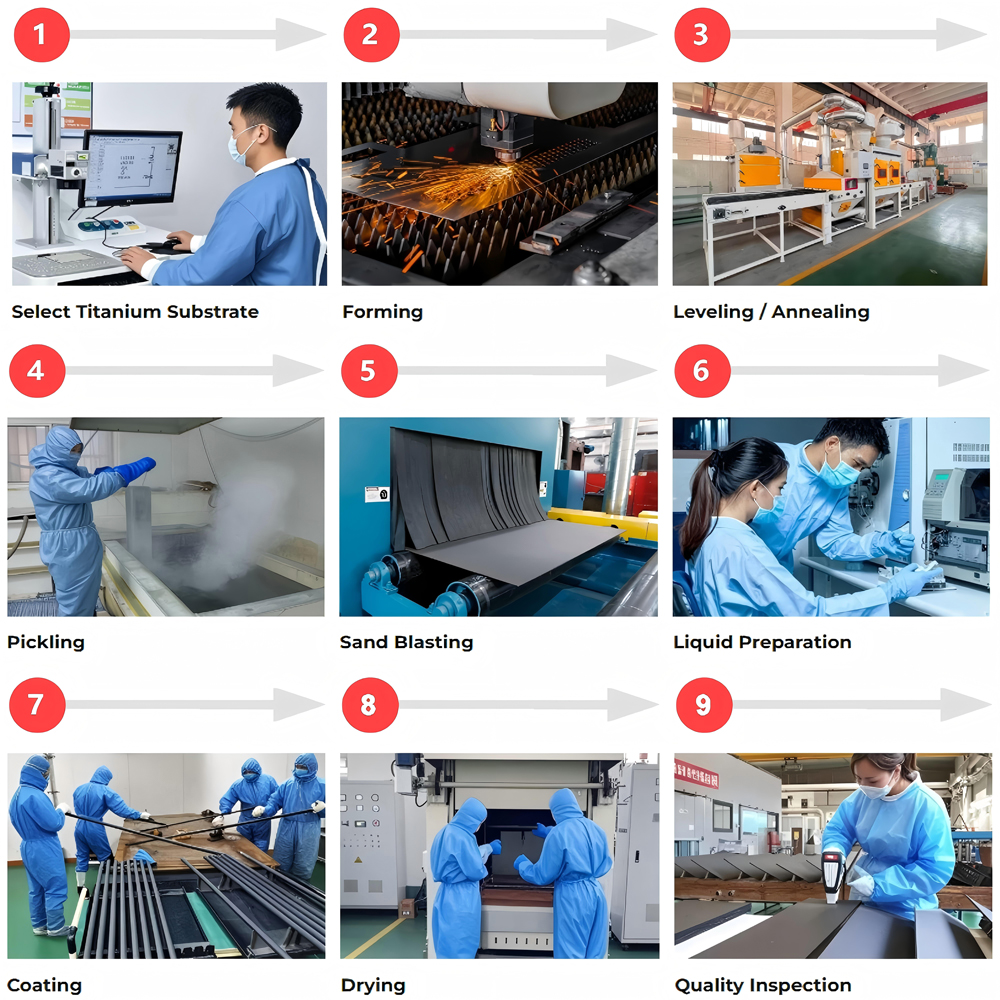
The core application of MMO titanium anodes in hydrometallurgy lies in leveraging the corrosion resistance of the titanium substrate and the catalytic activity of the MMO coating. By manipulating the electrochemical reaction, they achieve three key functions: metal ion extraction, impurity removal, and auxiliary reagent generation.
Titanium substrate: A dense TiO₂ passivation film (2-5nm thick) naturally forms on the surface of industrially pure titanium (Gr1/Gr2). This film exhibits extremely high chemical stability in strong acid and high-salt environments. In 98% concentrated sulfuric acid, the corrosion rate of titanium is less than 0.01mm/year; in 50% hydrochloric acid, the corrosion rate is less than 0.05mm/year, ensuring long-term resistance to the corrosive environment of hydrometallurgy.
MMO coatings: MMO coatings form a solid solution conductive network composed of precious metal oxides. This improves catalytic efficiency by lowering the overpotential of electrochemical reactions (for example, ruthenium-iridium coatings reduce the overpotential for chlorine evolution by 0.3-0.5V). Furthermore, the coating chemically bonds to the titanium substrate (forming a Ti-O-M bond, where M is a precious metal ion), resulting in strong adhesion (>50 MPa) and no flaking or dissolution at high current densities, ensuring long-term stable operation.
Electrolytic Deposition
This is the core application of MMO titanium anodes in hydrometallurgy. They are used to extract pure metals from electrolytes (electrodeposition) or purify crude metals (electrorefining). Taking copper electrorefining as an example, the specific mechanism is as follows:
Anodic Reaction: In a sulfuric acid-copper sulfate electrolyte, using an iridium-tantalum-based MMO titanium anode (oxygen-evolving type), the oxygen evolution reaction occurs at the anode: 2H₂O – 4e⁻ → O₂↑ + 4H⁺. The H+ generated by the oxygen evolution reaction replenishes hydrogen ions in the electrolyte, maintaining a stable pH (typically controlled between 1.8 and 2.2).
Cathode Reaction: At the cathode (copper or stainless steel plate), a copper ion reduction reaction occurs: Cu²+ + 2e⁻ → Cu↓. Copper ions are deposited on the cathode surface, forming high-purity cathode copper (purity exceeding 99.995%). Because the MMO anode eliminates the release of impurity ions, the electrolyte purity is higher, and the physical properties of the cathode copper (such as density and ductility) are significantly improved.
Current Efficiency: The MMO coating’s low overpotential (oxygen evolution overpotential is 0.2-0.3V lower than that of lead alloy anodes) reduces the cell voltage (from the traditional 0.35V to below 0.25V), reducing energy consumption at the same current. Furthermore, the coating’s high conductivity ensures more uniform current distribution, reduces “dendrite” on the cathode surface, and improves current efficiency (from 95% to over 97%).
During nickel electrodeposition in a chloride system, the ruthenium-iridium MMO titanium anode undergoes a chlorine evolution reaction: 2Cl⁻ – 2e⁻ → Cl₂↑. The generated chlorine can be recycled for chloride leaching of nickel minerals, completing a closed-loop “electrolysis-leaching” process, reducing chlorine procurement costs while also avoiding the environmental risks of chlorine leaks.
Leaching Principle
In the leaching and purification stages of hydrometallurgy, MMO titanium anodes catalytically generate oxidants (such as oxygen and chlorine) to achieve mineral leaching or impurity removal. The specific mechanism is as follows:
Assisted Leaching (using chloride leaching as an example): In chloride leaching of precious metals (gold and silver), the ruthenium-iridium MMO titanium anode electrolyzes sodium chloride solution to generate chlorine: 2Cl⁻ – 2e⁻ → Cl₂↑. The chlorine reacts with water to form hydrochloric acid and hypochlorous acid (Cl₂ + H₂O ⇌ HCl + HClO). Hypochlorous acid further oxidizes gold to form soluble chloroauric acid (Au + 3HClO + HCl → HAuCl₄ + 3H₂O), enabling gold dissolution and extraction. Compared to traditional chlorine gas flow, the MMO anode generates chlorine in situ, increasing utilization from 60% to over 90%, without the risk of chlorine leakage.
Solution purification (for example, iron removal): In copper and zinc leachates, Fe²+ is the primary impurity, affecting the purity of subsequent electrolytic products. It must be oxidized to Fe³+ and then separated by precipitation as iron hydroxide. Using a tin-antimony or iridium-tantalum-based MMO titanium anode, electrolysis in a dilute sulfuric acid system produces oxygen: 2H₂O – 4e⁻ → O₂↑ + 4H⁺. Oxygen oxidizes Fe²+ to Fe³+ (4Fe²+ + O₂ + 4H+ → 4Fe³+ + 2H₂O). After adjusting the pH to 3-4, Fe³+ hydrolyzes to form ferric hydroxide precipitate (Fe³+ + 3H₂O → Fe (OH)₃↓ + 3H+). The high oxygen evolution efficiency of the MMO anode enables an Fe²+ oxidation rate exceeding 99%, without the introduction of impurity ions.
Types of MMO Titanium Anodes
Hydrometallurgical processes are complex and diverse—from sulfuric acid systems for copper electrolysis to chloride systems for nickel and cobalt extraction, and from low-current-density purification to high-current-density electrodeposition—and the requirements for anode corrosion resistance, catalytic activity, and current-carrying capacity vary significantly.
The coating composition directly determines the anode’s corrosion resistance, catalytic selectivity, and applicable system, and is a key indicator for matching hydrometallurgical processes. Main types can be categorized as chlorine-evolving, oxygen-evolving, and strong-acid-resistant.
Ruthenium-Iridium Coated Titanium Anodes
Using ruthenium dioxide (RuO₂) as the core active ingredient, the anode is doped with 10%-30% iridium dioxide (IrO₂) to optimize stability. The coating thickness is controlled at 10-15μm, and the precious metal loading is 15-25g/m². Its core advantage is its efficient catalytic chloride ion oxidation, achieving a chlorine evolution current efficiency exceeding 95% in chloride systems (such as nickel chloride and cobalt chloride electrolytes). It also offers excellent resistance to chloride corrosion, withstanding chloride ion concentrations exceeding 100g/L and acidic environments with a pH range of 1-6. Its maximum current density reaches 3000A/m².
Iridium-Tantalum Coated Titanium Anode
Based on iridium dioxide (IrO₂), it is doped with 30%-50% tantalum pentoxide (Ta₂O₅) to form a solid solution coating with a thickness of 8-12μm and a precious metal loading of 20-35g/m². Its core advantages are strong acid resistance and high oxygen evolution stability. In oxygen-containing acid systems such as sulfuric acid and nitric acid, its oxygen evolution overpotential is as low as 1.4V. It can withstand 60% sulfuric acid concentrations and temperatures of 80°C, with a maximum current density of 12,000A/m². There is no risk of coating peeling during long-term use. Suitable for hydrometallurgy in sulfuric acid systems.
Tin-Antimony Coated Titanium Anode
Composed primarily of tin dioxide (SnO₂), this anode is doped with 5%-10% antimony trioxide (Sb₂O₃) to improve conductivity. The coating thickness is 15-20μm. Its cost is only one-third to one-half that of a ruthenium-iridium anode. Its core advantage is its resistance to strong oxidizing acid corrosion, including concentrated nitric acid and chromic acid. Its stable performance at low current densities (<500A/m²) makes it suitable for cost-sensitive weak acid or low current applications.
Plate-Shaped MMO Titanium Anode
This anode is 2-5mm thick and can be customized in sizes from 500×1000mm to 2000×3000mm. Its simple structure and easy installation make it the most commonly used anode in hydrometallurgy. Suitable for large electrolytic cells (such as copper and zinc electrolytic cells), these anodes can be installed individually or in groups, with electrolysis efficiency controlled by adjusting the spacing between anodes.
Mesh MMO Titanium Anodes
Made of titanium wire (1-3mm diameter) welded into a grid pattern, with mesh sizes ranging from 5×5mm to 20×20mm. They offer a surface area 3-5 times greater than plate anodes and improve current distribution uniformity by 40%. They are suitable for high-current-density electrodeposition (such as nickel-cobalt electrodeposition), reducing concentration polarization and increasing metal deposition rates.
Tubular MMO Titanium Anodes
Made of seamless titanium tubes (20-100mm diameter, 2-5mm wall thickness), they can be used individually or in series, making them suitable for pipeline leaching or circulating electrolysis systems. For example, in precious metal chloride leaching, tubular ruthenium-iridium anodes are installed within the reaction tube. Electrolysis of chloride solutions generates chlorine, enabling an immediate reaction between chlorine and minerals, improving leaching efficiency. In wastewater treatment, tubular anodes enable circulating electrolysis of the electrolyte, enhancing impurity removal.
Customized MMO Titanium Anodes
Customized to suit specific equipment structures, such as curved anodes (suitable for circular electrolytic cells), slot anodes (suitable for continuous electrodeposition production lines), and filament anodes (suitable for small precious metal recovery equipment). A custom-made 1mm diameter ruthenium-iridium filament anode for a precious metals recycling company achieves selective gold deposition in a micro-electrolytic cell, achieving a recovery rate of 99.9% and occupying only one-fifth the floor space of traditional equipment.
Related products
-
Titanium Fasteners
Custom Manufacturing Titanium Screws
-
Titanium Fasteners
Motorcycle Titanium Bolts Pin
-
Titanium Fasteners
CNC Machining Titanium Fasteners
-
Titanium Fasteners
Anodized Titanium Screws
-
Titanium Fasteners
Titanium Screws and Bolts
-
Titanium Fasteners
Colored Titanium Washers
-
Titanium Fasteners
Colored Motorcycle Titanium Bolts
-
Titanium Fasteners
Colored Titanium Nuts