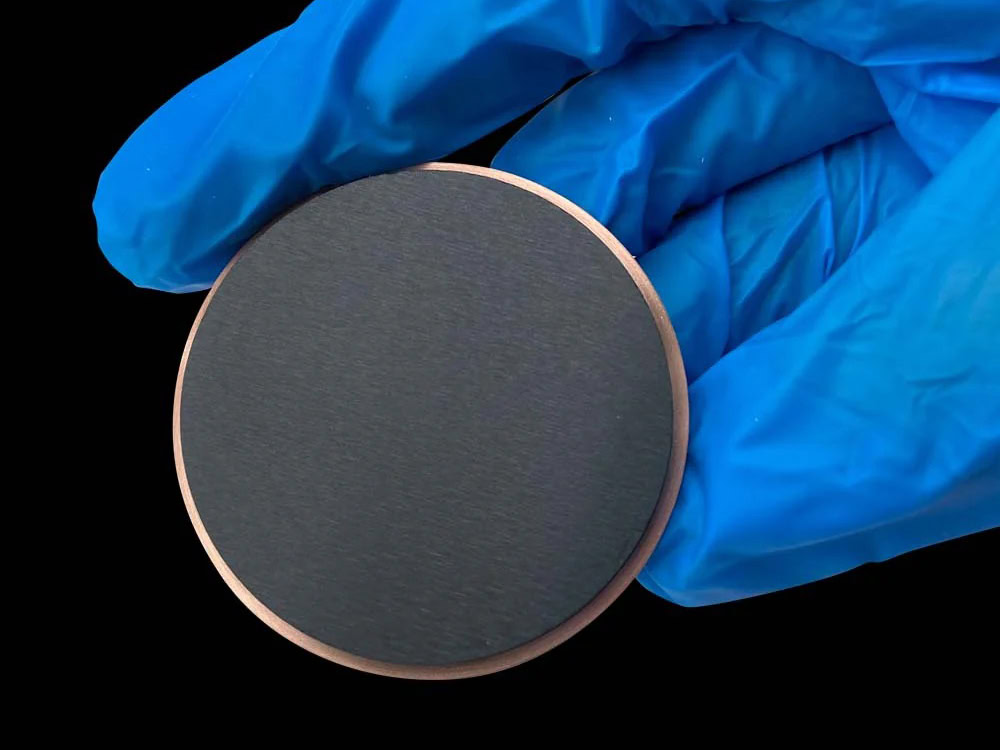
MMO Titanium Anode For Perchlorate
Certified: CE & SGS & ROHS
Shape: Requested
Diameter: Customized
Drawings: STEP, IGS , X_T, PDF
Shipping: DHL, Fedex, or UPS & Ocean Freight

20+ YEARS EXPERIENCE SENIOR BUSINESS MANAGER
Ask Michin For What You Want?
Perchlorates are a key class of inorganic chemical products. Potassium perchlorate is a core raw material for the manufacture of explosives, rocket propellants, and airbag explosives. Sodium perchlorate is a highly effective herbicide. Magnesium perchlorate is an excellent dehydrating agent with applications in strategic sectors such as energy, agriculture, and military. Currently, the mainstream industrial production of perchlorates is through the electrolysis of chlorate solutions. The core technical bottleneck lies in the selection of anode materials—the electrodes must be able to withstand high-potential oxidizing environments while also possessing excellent catalytic activity, corrosion resistance, and long life.
Titanium-based metal oxide-coated anodes (MMO titanium anodes, also known as DSA anodes) combine high catalytic activity, strong corrosion resistance, and dimensional stability. They have revolutionized the chlor-alkali and chlorate electrolysis industries and are now the preferred anode material for perchlorate production.
| Technical Measurement | Performance |
| Coating Element | Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂),Platinum |
| Substrate Material | Titanium Gr1 or Gr2 |
| Titanium Anode Shape | Customized Plate/Mesh/Tube/Rod/Wire/Disc |
| Coating Thickness | 8~20 μm |
| Coating Uniformity | 90% min. |
| Current Density | ≤ 20000 A/m² |
| Operating Voltage | ≤ 24V |
| PH Range | 1~14 |
| Temperature | < 80 °C |
| Fluoride Ion Content | < 50 mg/L |
| Warranty | More than 5 years |
Working Principle of the MMO Titanium Anode
Perchlorate production utilizes electrolysis of chlorate solutions. The MMO titanium anode, through its unique electrode structure and catalytic properties, drives the targeted oxidation of chlorate ions to perchlorate. This involves the coordinated action of charge transfer and chemical reactions at the electrode interface.
Electrochemical Reaction
The overall reaction for perchlorate synthesis is a coupled process of chlorate ion oxidation at the anode and water reduction at the cathode. The core function of the MMO titanium anode is to catalyze the anodic oxidation reaction.
Chlorate Preferred Oxidation: Chlorate ions (ClO₃⁻) first gain electrons on the MMO coating surface, becoming activated to form intermediate ClO₃ radicals. These radicals then react with water molecules to form perchlorate (ClO₄⁻). The reaction equation is: ClO₃⁻ + H₂O → ClO₄⁻ + 2H⁺ + 2e⁻. The standard electrode potential is 1.9V. Active ingredients such as IrO₂ in the MMO coating can lower the activation energy of this reaction, allowing it to proceed smoothly at a lower overpotential.
The preferential water oxidation: Water molecules first discharge on the anode surface to generate reactive oxygen atoms (O). These reactive oxygen atoms then combine with ClO₃⁻ in the solution to form ClO₄⁻. The reactions are: H₂O → O + 2H⁺ + 2e⁻, ClO₃⁻ + O → ClO₄⁻. This pathway is more likely to occur at high potentials. The high oxygen overvoltage characteristic of the MMO coating suppresses excessive oxygen evolution, ensuring that reactive oxygen species preferentially participate in the chlorate oxidation reaction, thereby reducing energy waste.
The cathode reaction primarily involves the reduction of hydrogen ions to generate hydrogen gas: 2H⁺ + 2e⁻ → H₂↑. The hydrogen produced in this reaction does not react with the anode product, eliminating the need for a diaphragm in the electrolyzer and simplifying the equipment structure.
MMO Coating Catalysis
The titanium substrate itself has poor electrical conductivity and is easily passivated. The MMO coating achieves efficient catalysis through the following mechanisms.
Electron Conduction: The precious metal oxides (such as IrO₂ and RuO₂) in the coating possess excellent electrical conductivity, effectively reducing electrode resistance and forming an electron conduction path from the titanium substrate to the electrolyte, thus addressing the titanium substrate’s insufficient electrical conductivity.
Active Site Enrichment Effect: The coating forms a porous structure through high-temperature sintering, providing a large number of electrochemically active sites. This significantly improves the adsorption and activation efficiency of ClO₃⁻ on the electrode surface, accelerating the reaction rate.
Stability Guarantee: The dense oxide layer formed by the coating isolates the electrolyte from direct contact with the titanium substrate, preventing titanium oxidation to form TiO₂, which increases contact resistance, while also preventing electrode failure caused by substrate corrosion.
Wstitanium’s Advantages
As a renowned manufacturer of MMO titanium anodes in China, Wstitanium has developed customized electrode products specifically for the demanding conditions of perchlorate production.
(I) Titanium Substrate
Substrate quality directly determines electrode life. Wstitanium uses Gr1 pure titanium as its substrate material. This material offers excellent corrosion resistance and mechanical strength, while also preventing substrate embrittlement caused by hydrogen diffusion during electrolysis. A combined sandblasting and pickling process creates a uniform roughness (Ra 1.5-3.0μm) on the titanium surface, improving the adhesion between the coating and the substrate by over 40% and effectively preventing anode passivation caused by coating flaking.
(II) Coating
The coating is prepared using a proprietary multi-component oxide formula. Ta₂O₅ is introduced as a stabilizer into the traditional IrO₂-TiO₂ system, significantly improving the coating’s oxidation resistance and wear resistance. By precisely controlling the brush coating and sintering parameters (sintering temperature 450-500°C, holding time 15-20 minutes), the coating thickness is precisely controlled to 10-12μm, with a coating uniformity of ≥85%, ensuring consistent catalytic activity across the electrode surface.
(III) Excellent Electrochemical Performance
The Wstitanium MMO titanium anode has an oxygen release potential of ≤1.8V, closely matching the reaction potential of perchlorate synthesis and effectively suppressing the oxygen evolution side reaction. Under typical operating conditions (current density 3kA/m², temperature 45°C), the current efficiency reaches over 92%, exceeding that of PbO₂ anodes (87%-89%) and conventional MMO anodes (88%-90%).
Thanks to its low overpotential and optimized coating structure, the electrode operating voltage is significantly reduced. Compared with graphite anodes, the cell voltage can be reduced by 0.7-1.0V, and DC power consumption can be reduced by 15%-20%. Based on an annual production capacity of 10,000 tons of sodium perchlorate, this can save approximately 3 million kWh of electricity annually.
(IV) Customization
To address the production differences of different perchlorate products (such as sodium perchlorate and potassium perchlorate), Wstitanium can provide customized solutions. For the high-concentration electrolyte conditions of potassium perchlorate production, the Ta₂O₅ content in the coating is optimized to enhance corrosion resistance. For continuous sodium perchlorate electrolysis, mesh or tubular electrode structures are designed to improve mass transfer efficiency. Electrode dimensions can be customized to suit electrolytic cell specifications, enabling stable production of small electrodes ranging from 100×200 mm to large electrodes several meters long.
MMO Titanium Anode Types
The core difference between MMO titanium anodes lies in their coating systems. Different oxide coatings exhibit significant differences in catalytic activity, oxidation resistance, and applicable operating conditions.
Iridium MMO Titanium Anode
Iridium-based coatings use IrO₂ as the primary active ingredient, typically combined with oxides such as TiO₂ and Ta₂O₅ to form a composite coating system. They are the most widely used type in perchlorate production. The coating thickness is typically controlled between 8 and 15 μm. By optimizing the ratio of IrO₂ to auxiliary oxides, the balance between catalytic activity and life can be further improved.
Platinum MMO Titanium Anode
Platinum-based coatings utilize platinum or platinum group metal oxides as the active layer, deposited directly on the titanium substrate or bonded via a transition layer. Their greatest strengths are their extremely high catalytic activity and low oxygen evolution overpotential, enabling electrolysis at lower cell voltages and significantly reducing power consumption.
Ruthenium-Iridium MMO Titanium Anode
The ruthenium-iridium composite coating uses RuO₂ and IrO₂ as dual active components. By adjusting the ratio of the two, a balanced chlorine and oxygen evolution performance is achieved.
Related products
-
Titanium Fasteners
Titanium Fasteners Manufacturer and Supplier
-
Titanium Fasteners
Titanium Bolts Supplier
-
Titanium Fasteners
Titanium Fasteners For Automotive
-
Titanium Fasteners
CNC Machining Titanium Fasteners
-
Titanium Fasteners
Anodized Titanium Nut
-
Titanium Fasteners
Colored Titanium Washers
-
Titanium Fasteners
Colored Automotive Titanium Bolts
-
Titanium Fasteners
Colored Gr5 Manifold Titanium Bolts