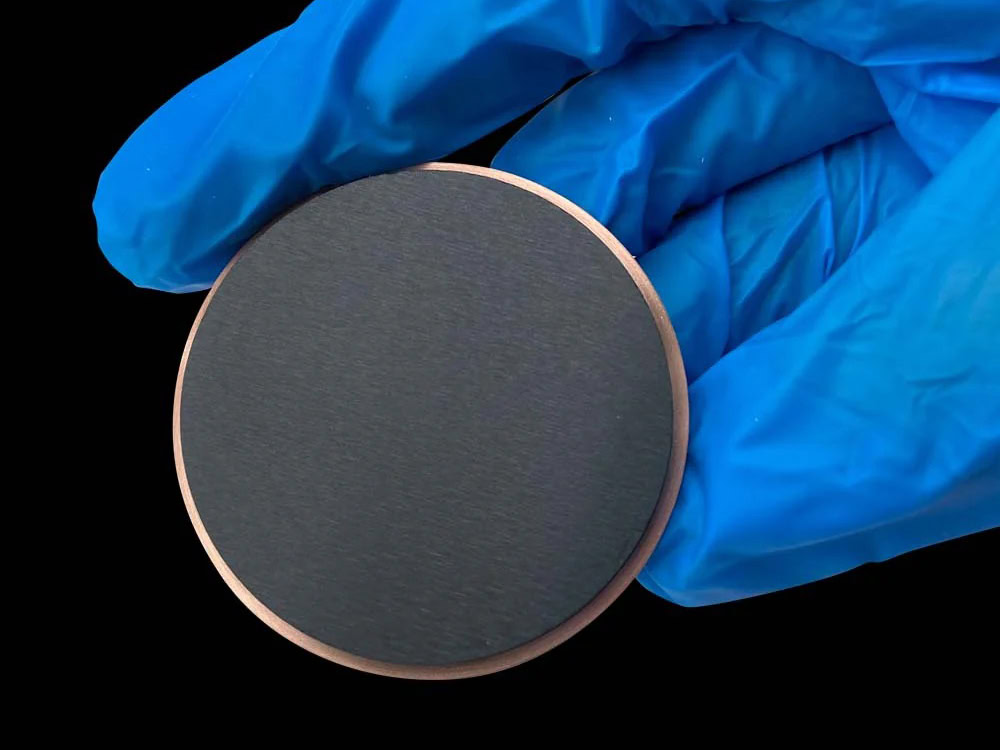
MMO Titanium Anode For Nitrogen
Certified: CE & SGS & ROHS
Shape: Requested
Diameter: Customized
Drawings: STEP, IGS , X_T, PDF
Shipping: DHL, Fedex, or UPS & Ocean Freight

20+ YEARS EXPERIENCE SENIOR BUSINESS MANAGER
Ask Michin For What You Want?
Nitrogen compound pollution has become a core challenge in global water management. It encompasses various forms, including ammonia nitrogen, nitrates, and nitrites, posing a serious threat to ecosystems and human health. Traditional nitrogen compound treatment technologies, such as biological denitrification, are limited by long reaction cycles, poor adaptability to low temperatures, and high sludge production. Chemical precipitation methods are prone to secondary pollution and struggle to meet current stringent environmental emission requirements.
MMO titanium anodes (titanium-based metal oxide-coated anodes) utilize a high-purity titanium substrate coated with a composite coating of precious metal oxides, such as ruthenium and iridium. They combine excellent electrocatalytic activity, corrosion resistance, and stability. Their application in nitrogen compound treatment achieves efficient pollutant degradation and is considered a key technological path to addressing the complex issue of nitrogen compound pollution.
| Technical Measurement | Performance |
| Coating Element | Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂),Platinum |
| Substrate Material | Titanium Gr1 or Gr2 |
| Titanium Anode Shape | Customized Plate/Mesh/Tube/Rod/Wire/Disc |
| Coating Thickness | 8~20 μm |
| Coating Uniformity | 90% min. |
| Current Density | ≤ 20000 A/m² |
| Operating Voltage | ≤ 24V |
| PH Range | 1~14 |
| Temperature | < 80 °C |
| Fluoride Ion Content | < 50 mg/L |
| Warranty | More than 5 years |
Pollution Hazards of Nitrogen Compounds
Agricultural production is the largest source of nitrogen compound emissions. Approximately 30%-50% of nitrogen fertilizer applied to fertilizers is not absorbed by crops and instead enters water bodies through surface runoff and soil seepage, contributing primarily to ammonia nitrogen and nitrate pollution. Chemical, pharmaceutical, and food processing industries are major contributors to industrial nitrogen pollution. Fertilizer and dye plants produce wastewater with high concentrations of ammonia nitrogen (up to thousands of mg/L). Nitrogen compounds in urban sewage primarily originate from human metabolic products and detergents. Nitrogen compound pollution accumulates through the food chain and spreads through ecological cycles, creating multi-layered hazards:
Damage to aquatic ecosystems: High concentrations of nitrogen compounds lead to eutrophication of water bodies, triggering explosive growth of algae such as cyanobacteria, resulting in “algal blooms” or “red tides.” The decomposition of dead algae consumes large amounts of dissolved oxygen, suffocating fish and other aquatic organisms and disrupting the ecological balance of the water body.
Threats to human health and safety: Nitrates in drinking water are converted to nitrites in the human digestive tract, which then bind to hemoglobin to form methemoglobin, leading to tissue hypoxia and “blue baby syndrome,” a particularly serious threat to infants and young children. Nitrates can also react with amines in the body to form nitrosamines, a potent carcinogen, increasing the risk of digestive system cancers. Nitro compounds in industrial wastewater are highly toxic and can cause liver and kidney damage through skin contact or drinking contaminated water.
Increased burden on environmental management: Nitrogen compound pollution is cumulative and migratory. Once in the soil, it reduces soil fertility and affects crop quality. Seeping into groundwater, it forms a regional pollution plume, with remediation costs reaching hundreds of dollars per cubic meter. Furthermore, nitrogenous waste gases and nitrogen compound wastewater transform through the atmosphere-water cycle, further expanding the scope of pollution and causing “secondary pollution.”
Working Principle of the MMO Titanium Anode
The MMO titanium anode achieves efficient nitride removal through a dual mechanism of electrochemical oxidation and catalytic conversion. Its core principle is to leverage the high catalytic activity of the coating to trigger a series of redox reactions on the electrode surface, converting toxic and harmful nitrides into harmless substances.
(I) Direct Electrochemical Oxidation
Under the influence of an electric field, nitride molecules are directly adsorbed onto active sites on the surface of the MMO titanium anode (such as RuO₂ and IrO₂ grains), where they are oxidized and degraded through electron transfer. For ammonia nitrogen, a dehydrogenation reaction occurs on the anode surface, first converting it to the intermediate product hydrazine (N₂H₄), which is then oxidized to nitrogen gas (N₂). The core reaction equation is: 2NH₃ – 6e⁻ = N₂↑ + 6H⁺. This process requires no additional oxidant and offers high reaction selectivity, with nitrogen yields exceeding 85%. For aromatic nitrogen compounds such as nitrobenzene, direct oxidation cleaves the C-N bond on the benzene ring, converting the nitro group (-NO₂) to nitrate (NO₃⁻), which is then further oxidized to nitrogen gas, achieving simultaneous toxicity elimination and nitrogen removal.
(II) Indirect Electrochemical Oxidation
During the electrolysis process, the MMO titanium anode oxidizes chloride ions (Cl⁻) and water molecules in the water, generating highly oxidizing active substances. When chloride ions are present in the wastewater, a chlorine evolution reaction occurs on the anode surface: 2Cl⁻ – 2e⁻ = Cl₂↑. The chlorine gas further reacts with water to form hypochlorous acid (HClO) and hypochlorite (ClO⁻). These chlorine-based oxidants can rapidly oxidize ammonia nitrogen to nitrogen gas, increasing the reaction rate by 3-5 times compared to direct oxidation. In a chloride-free system, water molecules are oxidized at the anode to produce reactive oxygen species (・OH, O₂⁻, and other free radicals). The hydroxyl radical (・OH) has a redox potential as high as 2.8V and can non-selectively degrade nitrates, nitrites, and other compounds, converting them into nitrogen gas or nitrates.
(III) Synergistic Electrocatalytic Conversion
The precious metal oxides in the MMO coating form a solid solution structure with valve metal oxides (such as IrO₂-Ta₂O₅ and RuO₂-TiO₂). This unique electron conduction property reduces the reaction activation energy and promotes the selective conversion of nitrides. When treating nitrate wastewater, the catalytic sites on the anode surface can regulate the reaction pathway, inhibiting the side reaction of over-oxidation to nitrate and promoting the conversion of nitrate to nitrite via a two-step electron transfer process, followed by further reduction to nitrogen gas. Research has shown that Ir-Ta-based MMO anodes can increase the nitrogen conversion efficiency of nitrate to over 90%, significantly exceeding that of conventional electrodes. At the same time, the 10-nanometer TiO₂ passivation film formed on the surface of the titanium substrate can inhibit electrode corrosion and ensure the long-term stability of catalytic activity.
MMO Titanium Anode Types
Based on coating composition and catalytic properties, MMO titanium anodes suitable for nitride treatment are primarily classified into three categories.
(I) Ruthenium MMO Titanium Anodes
This type of electrode uses RuO₂ as the primary active ingredient, forming a 20-30μm thick porous coating on a titanium substrate via thermal decomposition. Its core advantage is its low chlorine evolution overpotential (140mV lower than that of a graphite anode at a current density of 1A/cm²). It can efficiently generate hypochlorous acid in chlorine-containing wastewater systems, making it particularly suitable for treating ammonia nitrogen with high chlorine content, such as municipal sewage and aquaculture wastewater.
(II) Iridium MMO Titanium Anodes
The IrO₂-Ta₂O₅/Ti anode, prepared via a sol-gel method using IrO₂ as the active component and Ta₂O₅ as a coating enhancer, is a star product in the oxygen evolution reaction field. IrO₂ has extremely high catalytic activity for oxygen evolution. The solid solution formed by Ta₂O₅ and IrO₂ significantly improves coating stability and inhibits the loss of active components. Its outstanding advantages include strong corrosion resistance and strong passivation resistance. It operates stably in a wide range of water bodies with a pH of 1-14, making it particularly suitable for treating highly acidic, high-concentration nitro compounds, such as chemical wastewater. When treating wastewater with a nitrobenzene concentration of 200 mg/L, this electrode achieved an 89% nitrobenzene removal rate and a 72% TOC removal rate within 3 hours at a current density of 25 mA/cm² and a temperature of 40°C. Furthermore, this type of electrode boasts a lifespan of over 6 years, making it the preferred electrode type for challenging nitrogen pollution control.
(III) Platinum MMO Titanium Anode
The Pt-IrO₂/Ti composite electrode, formed by introducing platinum (Pt) into the coating, combines high catalytic activity with an exceptionally long lifespan. The addition of platinum reduces the activation energy for nitride oxidation, improving reaction selectivity and enabling efficient conversion of nitrates to nitrogen gas at low current densities. This type of electrode is suitable for applications requiring extremely high water quality, such as deep nitrogen removal from drinking water and ultrapure water production for the electronics industry. After treatment, nitride concentrations in treated water can be reduced to below 0.5 mg/L, without the risk of heavy metal dissolution. An application case study at an electronics factory demonstrated that a Pt-IrO₂/Ti anode treated process wastewater with a nitrate concentration of 50 mg/L achieved a 99% removal rate at a current density of 5 mA/cm², with the effluent meeting electronic-grade pure water standards. However, due to its high precious metal content, the cost is 3-5 times that of ruthenium-based electrodes, making it primarily used in high-end treatment scenarios.
Related products
-
Titanium Fasteners
Anodized Titanium Screws
-
Titanium Fasteners
Titanium Fasteners Manufacturer and Supplier
-
Titanium Products
Titanium Fasteners For Motorcycles
-
Titanium Fasteners
Custom Gr5 Titanium Fasteners
-
Titanium Fasteners
CNC Machining Titanium Fasteners
-
Titanium Fasteners
Colored Titanium Washers
-
Titanium Fasteners
Colored Bicycle Titanium Bolts
-
Titanium Fasteners
Colored Gr5 Manifold Titanium Bolts