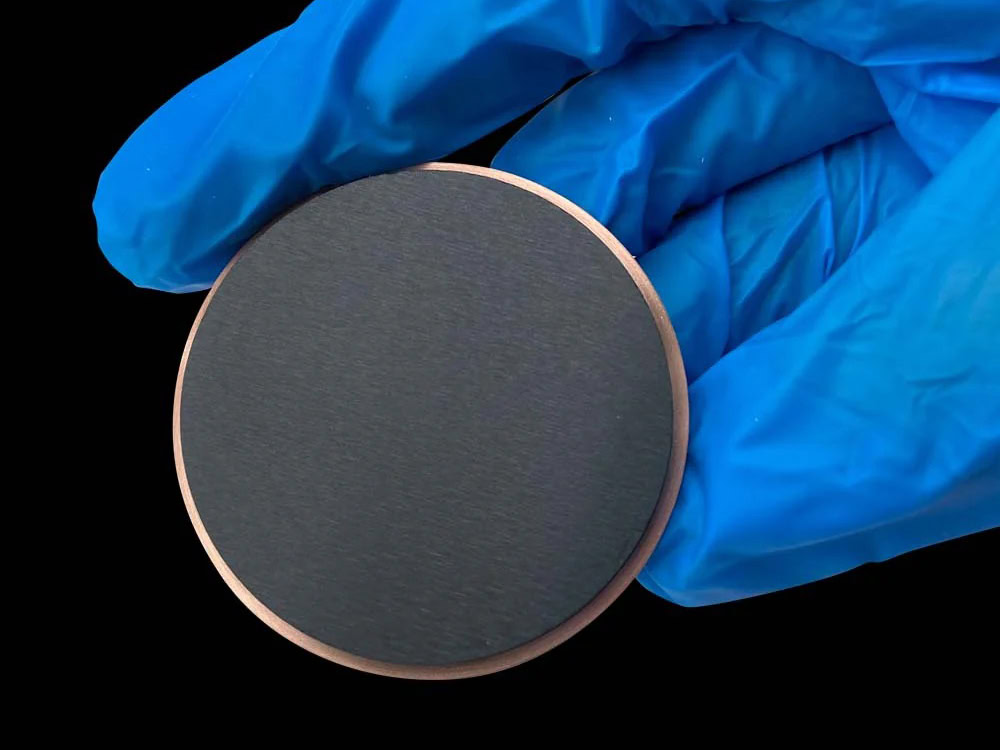
Platinum Coating Titanium Anode
Certified: CE & SGS & ROHS
Shape: Requested
Diameter: Customized
Drawings: STEP, IGS , X_T, PDF
Shipping: DHL, Fedex, or UPS & Ocean Freight

20+ YEARS EXPERIENCE SENIOR BUSINESS MANAGER
Ask Michin For What You Want?
In the field of electrochemistry, electrodes serve as the core carriers of energy conversion and material reactions. Their performance directly determines efficiency, quality, and technical stability. With the rapid development of industries such as new energy, environmental protection, and electroplating, traditional electrode materials such as graphite and lead alloys have gradually exposed shortcomings such as poor corrosion resistance, short lifespan, high energy consumption, and the generation of secondary pollution.
Pt-titanium anodes, leveraging the excellent electrochemical activity of platinum and the high strength and corrosion resistance of the titanium substrate, have become a key material for addressing electrode failure under harsh operating conditions. The titanium substrate not only possesses excellent mechanical strength and performance, adapting to diverse electrode configurations (such as plate, mesh, tubular, and filamentary), but also forms a dense oxide film (TiO₂) in strong acid, alkaline, and highly oxidizing environments, effectively protecting against corrosion from corrosive media. The platinum coating, acting as the “active center” for electrochemical reactions, exhibits extremely high overpotential stability for oxygen and chlorine evolution, significantly reducing electrochemical reaction energy consumption while also preventing platinum-titanium anode dissolution during the reaction.
The application of platinum-titanium anodes began in the chlor-alkali industry in the mid-to-late 20th century. With the iteration of manufacturing technology, its application scenarios have gradually expanded to electroplating (such as gold plating, silver plating, nickel plating), water electrolysis (hydrogen production, oxygen production), sewage treatment (electrocatalytic oxidation degradation of pollutants), metal electrolytic refining (such as copper, nickel, and cobalt purification) and other fields.
| Technical Measurement | Performance |
| Coating Element | Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂),Platinum |
| Substrate Material | Titanium Gr1 or Gr2 |
| Titanium Anode Shape | Customized Plate/Mesh/Tube/Rod/Wire/Disc |
| Coating Thickness | 8~20 μm |
| Coating Uniformity | 90% min. |
| Current Density | ≤ 20000 A/m² |
| Operating Voltage | ≤ 24V |
| PH Range | 1~14 |
| Temperature | < 80 °C |
| Fluoride Ion Content | < 50 mg/L |
| Warranty | More than 5 years |
Advantages of Platinum-Titanium Anodes
Compared to traditional electrode materials, platinum-titanium anodes exhibit significant advantages in performance, cost-effectiveness, and environmental friendliness. These advantages stem from their “platinum coating + titanium substrate” composite structure.
1. Excellent Corrosion Resistance
In the electrochemical industry, electrodes are often exposed to strong acids (such as sulfuric acid, hydrochloric acid, and nitric acid), strong bases (such as sodium hydroxide), high salts (such as sodium chloride and magnesium chloride), or highly oxidizing environments (such as hypochlorous acid and hydrogen peroxide). Traditional electrodes (such as graphite and lead alloys) are prone to corrosion, dissolution, or structural damage, resulting in a short electrode life (typically only a few months to a year), requiring frequent replacement, increasing downtime costs and maintenance workload.
The corrosion resistance of platinum-titanium anodes stems from two key properties: First, the passivation effect of the titanium substrate—titanium quickly forms a dense oxide film (TiO₂) approximately 5-10 nm thick in corrosive media. This oxide film is extremely chemically stable, effectively isolating the corrosive medium from the substrate and preventing further oxidation of the titanium. Secondly, the platinum coating is chemically inert. Platinum is one of the most chemically stable precious metals. It does not dissolve in most acidic and alkaline environments from room temperature to high temperatures (≤600°C) and is resistant to corrosion by strong oxidizing ions such as Cl⁻, O₂, and H₂O₂.
2. Excellent Electrochemical Activity
The energy consumption of an electrochemical reaction is directly related to the electrode’s “overpotential.” The lower the overpotential, the lower the applied voltage required for the reaction, resulting in lower energy consumption. Traditional electrodes (such as lead alloys) have a high overpotential for oxygen evolution (typically 0.6-0.8V), resulting in a significant amount of electrical energy being wasted during the electrolysis process, converted into heat. However, the platinum coating exhibits extremely high electrocatalytic activity, significantly reducing the overpotential for key reactions such as oxygen and chlorine evolution. Taking water electrolysis as an example, the oxygen evolution overpotential of a platinum-titanium anode under alkaline conditions is only 0.2-0.3V. Compared to a lead alloy anode, this can reduce the cell voltage of the electrolyzer by 0.4-0.5V. For an electrolyzer with an annual hydrogen production capacity of 1000 Nm³, this can save approximately 1.2×10⁵ kWh of electricity, equivalent to approximately 40 tons of standard coal, annually. This not only reduces production costs but also carbon emissions. Furthermore, the platinum coating’s highly uniform activity prevents “hot spots” caused by excessively intense local reactions on the electrode surface, further improving electrolysis stability and reducing side reactions (such as the generation of impurity gases and metal ion dissolution).
3. Excellent Mechanical Properties
The electrode’s structural design must be tailored to the specific application scenario (e.g., electrolytic cell size, reaction flow distribution, and installation space). Traditional brittle materials (such as graphite) are difficult to process into complex shapes (e.g., thin-walled tubes or porous meshes) and are susceptible to breakage during installation and transportation. Titanium, on the other hand, offers excellent mechanical properties, with a tensile strength of 500-700 MPa and an elongation of approximately 15%-20%. Through conventional processing techniques such as stamping, welding, and cutting, it can be fabricated into a variety of structures, including plates, meshes, tubes, filaments, and spirals, to meet the needs of diverse operating conditions.
For example, in electrocatalytic oxidation equipment for environmental water treatment, porous mesh platinum-titanium anodes are required to increase the contact area between the wastewater and the electrode. Furthermore, the platinum coating exhibits a strong bond with the titanium substrate (adhesion exceeding 50 MPa) and resists detachment under conditions such as vibration and temperature fluctuations (from -50°C to 200°C).
4. Environmentally Friendly and Pollution-Free
Traditional electrodes are prone to secondary pollution during use. For example, trace amounts of lead ions dissolve from lead alloy anodes during the electrolysis process, entering the electrolyte or products (such as electroplated parts and drinking water), posing a threat to human health and the environment. Graphite anodes also undergo oxidative wear during the electrolysis process, producing graphite dust that contaminates the electrolyte and requires regular cleaning.
Platinum-titanium anodes fundamentally address this pollution problem. Firstly, the platinum dissolution rate is extremely low (in an acidic environment at room temperature, the annual dissolution rate is ≤0.1 mg/m²), eliminating heavy metal pollution. Secondly, after the electrodes are scrapped, platinum resources can be recovered through specialized technology (with a recovery rate exceeding 95%), achieving material recycling and aligning with the development concept of “green manufacturing.”
5. Long-Term Operational Stability
Traditional electrodes have a short lifespan and are prone to corrosion, requiring frequent downtime for replacement. This not only increases the workload for maintenance personnel, but also causes production interruptions and impacts productivity. Platinum-titanium anodes, with their long lifespan (typically 5-10 years) and high stability, significantly reduce maintenance frequency and downtime.
Taking a chlor-alkali plant as an example, traditional graphite anodes need to be replaced every one to two years. Each replacement requires three to five days of downtime, resulting in a production loss of approximately 10% to 15%. With the introduction of platinum-titanium anodes, replacement is required every five to eight years, reducing downtime to once every five years. This increases effective production time by approximately 10 to 15 days annually. Based on a profit of 200 yuan per ton of caustic soda, a plant with an annual production capacity of 100,000 tons of caustic soda can generate an additional profit of approximately 500,000 to 800,000 yuan. Furthermore, platinum-titanium anodes eliminate the need for frequent maintenance operations such as adjusting the electrode spacing and replenishing electrolyte, further reducing operational costs and improving production efficiency.
As a core material in the electrochemical industry, platinum-titanium anodes have successfully solved the pain points of short life, high energy consumption, and high pollution of traditional electrodes (graphite, lead alloys) by virtue of the “high activity and corrosion resistance of the platinum coating” and the “high strength and processability of the titanium substrate.” They have become a key support for the upgrading and development of industries such as chlor-alkali, electroplating, electrolysis of water to produce hydrogen, and environmentally friendly water treatment.
Related products
-
Titanium Fasteners
Custom Manufacturing Titanium Screws
-
Titanium Fasteners
Titanium Hexagon Head Bolts
-
Titanium Fasteners
Titanium Screws and Bolts
-
Titanium Fasteners
Colored Titanium Washers
-
Titanium Fasteners
Titanium Wheel Bolts
-
Titanium Fasteners
Colored Motorcycle Titanium Bolts
-
Titanium Fasteners
Colored Titanium Nuts
-
Titanium Fasteners
Burnt Titanium Wheel Bolt