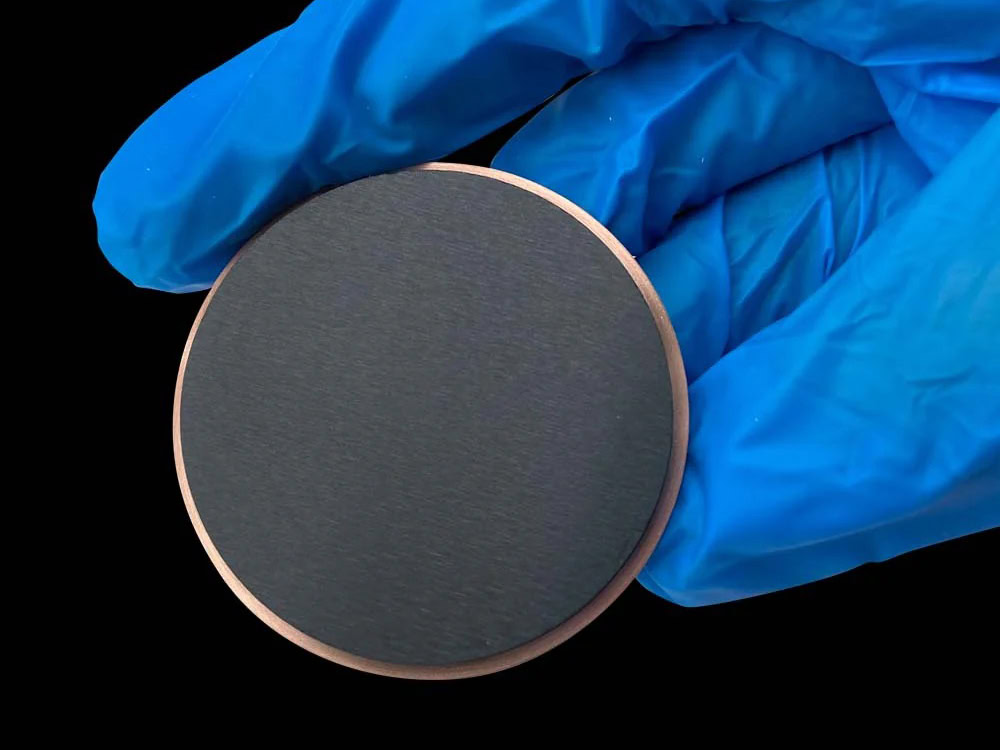
Ruthenium Iridium Titanium Anode
Certified: CE & SGS & ROHS
Shape: Requested
Diameter: Customized
Drawings: STEP, IGS , X_T, PDF
Shipping: DHL, Fedex, or UPS & Ocean Freight

20+ YEARS EXPERIENCE SENIOR BUSINESS MANAGER
Ask Michin For What You Want?
When current flows through an electrolyte solution, triggering a redox reaction, the performance of the anode directly determines reaction efficiency, product purity, and economic viability. In the electrochemical industry, electrode materials have evolved from graphite and lead-based alloys, ultimately leading to the development of the “excellent” ruthenium-iridium-titanium anode.
This composite electrode, based on industrial-pure titanium and coated with ruthenium-iridium oxide, perfectly balances catalytic activity, corrosion resistance, and mechanical stability, profoundly reshaping manufacturing methods in core areas such as the chlor-alkali industry, new energy development, and environmental governance.
| Technical Measurement | Performance |
| Coating Element | Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂),Platinum |
| Substrate Material | Titanium Gr1 or Gr2 |
| Titanium Anode Shape | Customized Plate/Mesh/Tube/Rod/Wire/Disc |
| Coating Thickness | 8~20 μm |
| Coating Uniformity | 90% min. |
| Current Density | ≤ 20000 A/m² |
| Operating Voltage | ≤ 24V |
| PH Range | 1~14 |
| Temperature | < 80 °C |
| Fluoride Ion Content | < 50 mg/L |
| Warranty | More than 5 years |
1. Excellent Electrochemical Performance
The core competitiveness of the ruthenium-iridium-titanium anode lies in its extremely low overpotentials for chlorine and oxygen evolution. Ruthenium oxide acts as an accelerator for the chlorine evolution reaction. It significantly reduces the reaction voltage in brine electrolysis, reducing power consumption per ton of caustic soda by 10%-20%. Iridium oxide optimizes the activity of the oxygen evolution reaction, reducing the overpotential to 0.25V in water electrolysis for hydrogen production, increasing hydrogen production efficiency by 40%-60%, and achieving hydrogen purity of 99.99%. This high catalytic efficiency results in significant current efficiency advantages, exceeding 95% in the chlor-alkali industry. In electroplating, the metal ion deposition rate can be controlled to within ±1%.
2. Extremely Corrosion Resistance
The titanium substrate is constructed from TA1/TA2 industrially pure titanium, which offers corrosion resistance far exceeding that of stainless steel and has a density only 60% of steel. It can operate stably and long-term in extreme acid and alkaline environments with a pH range of 0-14. After sintering at 500-600°C, the ruthenium-iridium oxide coating forms a tight bond with the substrate, achieving adhesion levels of ASTM D3359 Class B. In corrosive environments with chloride ion concentrations up to 5%, the coating exhibits an annual wear rate of only 0.07μm. By improving the coating formula with the addition of elements such as tantalum and tin, oxide dissolution and passivation are further delayed, enabling the anode to operate stably for over 4,000 hours under normal operating conditions, with a lifespan three to five times that of traditional lead anodes. With extreme optimization, this can be extended to over six years.
3. Excellent Dimensional Stability
The high strength of the titanium substrate ensures that the electrode does not deform or dissolve during electrolysis, with a gap change rate of less than 0.1% per year, providing a stable environment with millimeter-level precision for the reaction. Compared to traditional lead-based anodes, ruthenium-iridium-titanium anodes eliminate the risk of heavy metal dissolution contamination, completely eliminating the risk of excessive lead in products or water in applications such as electroplating and drinking water treatment. Furthermore, its modular design allows for customization in a variety of shapes, adapting to different electrolytic cell configurations. The precious metal recovery rate from used electrodes reaches 98%.
4. Significant Cost-Effectiveness
Despite using precious metal coatings such as ruthenium and iridium, the overall cost of ruthenium-iridium-titanium anodes is significantly lower than that of pure platinum anodes and traditional electrodes. Its material cost is only one-third to one-half that of pure platinum anodes, while its service life is several times that of lead anodes. Its low resistance reduces DC power consumption by 10%-20%. For example, a 2,000m³ swimming pool disinfection system consumes only 3,800kW·h annually, a 70% energy saving compared to ozone disinfection. Furthermore, the recycled value of used electrodes can reach 300-3,000 yuan per kilogram, reducing the total lifecycle cost by over 58% compared to traditional solutions, achieving a balance between short-term investment and long-term benefits.
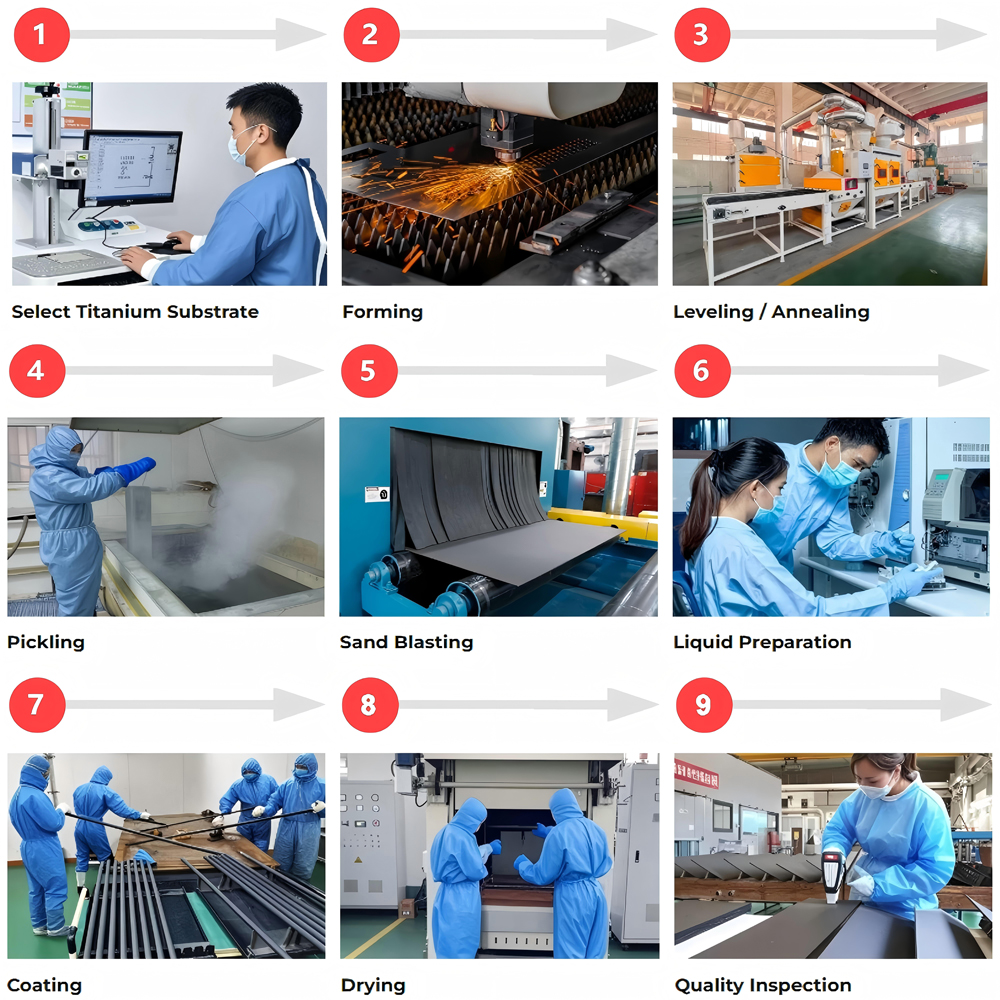
Manufacturing Ruthenium-Iridium-Titanium Anodes
The substrate is a key component in determining anode life and directly impacts the bonding strength between the coating and the titanium substrate. Sandblasting begins with high-speed spraying of diamond abrasive to create a roughened surface, increasing the specific surface area. This is followed by pickling and passivation, where the titanium substrate is immersed in a mixture of oxalic acid or hydrofluoric acid to remove the surface oxide layer and oil contamination while simultaneously creating a microscopic porous structure, increasing the coating’s adhesion by more than three times. Wstitanium also utilizes micro-arc oxidation technology, using a high voltage of 20,000V to create a nanoscale honeycomb structure on the titanium surface, further enhancing the coating’s adhesion. Electrolysis efficiency is increased to 95.2%.
1. Core Coating
Currently, the mainstream industry method for preparing ruthenium-iridium oxide coatings is thermal decomposition. This technique precisely controls temperature and atmosphere, enabling precise control of the coating’s composition and structure. First, a coating stock solution is prepared by dissolving precious metal precursors such as chlororuthenic acid and chloroiridic acid in a mixture of alcohol and hydrochloric acid. Metal salts such as tantalum and tin can be added as modifiers based on application requirements. The mother liquor is then evenly applied to the titanium substrate surface by brushing or spraying. After drying at 120°C to remove the solvent, the precursor is sintered in a muffle furnace at 500-600°C for 10-15 minutes, decomposing the precursor into oxides that chemically bond to the titanium substrate.
To achieve ideal performance, the coating undergoes multiple coating-sintering cycles, ultimately forming a uniform coating with a thickness of 0.5-20μm. Atomic layer deposition (ALD) technology has been introduced in high-end manufacturing, enabling nanoscale coating thickness control and forming a three-dimensional network structure that effectively prevents electrolyte penetration and reduces coating loss to just one-fifth of that achieved with traditional technologies. In some applications, a gradient coating design is employed, creating a three-layer structure consisting of a tantalum base layer, a tantalum oxide transition layer, and an iridium-ruthenium oxide top layer. This mitigates thermal expansion differences and reduces coating peeling rates to less than 0.5%.
2. Quality Inspection
The sintered anode undergoes post-processing, including cooling, cleaning, and performance testing. The coating is first slowly cooled in an inert atmosphere to prevent microcracks caused by thermal stress. Deionized water is then used to clean any remaining impurities from the surface, and activation treatment is performed if necessary to enhance catalytic activity. Quality inspections cover several key indicators: coating thickness is measured using an eddy current thickness gauge, ensuring an accuracy within ±0.1μm; adhesion is tested using the cross-hatch method, meeting ASTM D3359 Class B or higher; electrochemical performance is measured using linear sweep voltammetry, with a chlorine evolution overpotential below 0.1V and an oxygen evolution overpotential no greater than 0.25V. Furthermore, accelerated life testing is required, requiring continuous operation at a high current density of 3000A/m² for 1000 hours, with a coating loss rate below 0.1g/kA·h for delivery.
1. Chlor-alkali Industry
The chlor-alkali industry is the largest application area for ruthenium-iridium-titanium anodes. In the ion-exchange membrane process for caustic soda, ruthenium-iridium-titanium anodes catalyze the electrolysis of brine to produce caustic soda, chlorine, and hydrogen. Their low cell voltage increases annual production capacity per line by 10%, equivalent to reducing standard coal consumption by 3,000 tons per year. Data from a large chlor-alkali company shows that the adoption of ruthenium-iridium-titanium anodes has reduced electricity consumption per ton of caustic soda from 2,400 kWh to below 2,000 kWh, saving over 10 million yuan in annual electricity costs. The anode lifespan also exceeds three years.
2. New Energy Sector
In the field of hydrogen production through water electrolysis, ruthenium-iridium-titanium anodes, with their low oxygen evolution overpotential, have become core components of PEM electrolyzers, increasing hydrogen production efficiency to over 85%. In lithium extraction from lepidolite, ruthenium-iridium-titanium anodes are used for electrochemical leaching, increasing lithium leaching rates from 60% in traditional processes to over 90% without chemical contamination.
3. Water Treatment and Purification
In water treatment, ruthenium-iridium-titanium anodes can perform dual functions of disinfection and pollutant degradation. In swimming pool and drinking water disinfection, they electrolyze low-concentration brine to produce hypochlorous acid, which is 80 times more effective than traditional chlorine agents. They can kill 99.99% of E. coli within 30 seconds without producing carcinogenic byproducts such as chloroform. In industrial wastewater treatment, ruthenium-iridium-titanium anodes produce hydroxyl radical (・OH) concentrations three times higher than traditional electrodes, achieving COD removal rates exceeding 95% for difficult-to-degrade pollutants such as phenol and cyanide, enabling treated antibiotic wastewater to meet Class IV surface water standards.
4. Electroplating and Metallurgy
In the electroplating industry, ruthenium-iridium-titanium anodes, as insoluble anodes, completely eliminate the dissolution contamination problem associated with traditional lead anodes. In chrome and nickel plating processes, their uniform current distribution keeps coating thickness tolerances within ±0.5 microns, reducing burr defects by 60%. In the electroplating of precious metals such as gold and platinum, they provide a stable anode reaction interface, increasing coating purity to over 99.99%. In hydrometallurgy, they replace lead-based anodes in copper and zinc electrolytic refining, preventing lead ion contamination of the electrolyte and increasing cathode copper purity from 99.5% to 99.99%. The anode also boasts an anode lifespan of over two years.
5. Precision Manufacturing
In electronics manufacturing, ruthenium-iridium-titanium anodes are used in the electroplating of copper for 5G high-frequency copper-clad laminates. Their current density uniformity keeps copper layer thickness tolerances within ±0.5 microns, meeting the requirements of millimeter-wave signal transmission. In printed circuit board (PCB) through-hole plating, uniform copper plating can be achieved for through-holes with a depth-to-diameter ratio of 5:1, with a pass rate of over 99%.
Related products
-
Titanium Fasteners
Custom Manufacturing Titanium Screws
-
Titanium Fasteners
Titanium Bolts For Motorcycle
-
Titanium Fasteners
Titanium Flanged Hex Bolt
-
Titanium Fasteners
Titanium Fastener Hexagon Screw Nuts
-
Titanium Fasteners
Titanium Wheel Bolts
-
Titanium Fasteners
Colored Automotive Titanium Bolts
-
Titanium Fasteners
Colored Bicycle Titanium Bolts
-
Titanium Fasteners
Colored Titanium Nuts